Do Acids Or Bases React With Metals
Muz Play
Mar 31, 2025 · 5 min read
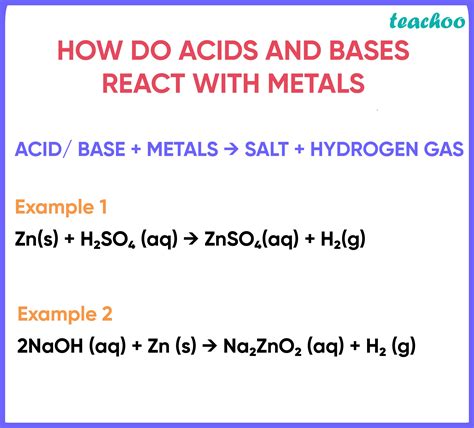
Table of Contents
Do Acids or Bases React with Metals? A Comprehensive Exploration
The reactivity of metals with acids and bases is a fundamental concept in chemistry, impacting various industrial processes and natural phenomena. While both acids and bases can react with certain metals, their mechanisms and the extent of their reactions differ significantly. This comprehensive article delves into the intricate details of these reactions, exploring the underlying principles, specific examples, and the factors influencing their outcome.
The Reactivity Series: A Guiding Principle
Understanding which metals react with acids and bases necessitates familiarity with the reactivity series, also known as the activity series. This series arranges metals in order of their decreasing reactivity, indicating their tendency to lose electrons and form positive ions (cations). Metals higher in the series are more reactive than those lower down. The reactivity series is crucial because it predicts which metals will undergo reactions with acids and bases.
Highly Reactive Metals (Top of the Series): These metals readily react with both acids and, in some cases, bases. Examples include alkali metals (like sodium, potassium, and lithium) and alkaline earth metals (like magnesium and calcium).
Moderately Reactive Metals (Middle of the Series): These metals react with acids but may require specific conditions or stronger acids to react with bases. Examples include zinc, iron, and aluminum.
Less Reactive Metals (Bottom of the Series): These metals generally do not react with acids or bases under normal conditions. Examples include copper, silver, and gold.
Reactions of Metals with Acids
The most common reaction of metals with acids involves the displacement reaction, where the metal displaces hydrogen from the acid. This reaction is typically exothermic, meaning it releases heat. The general equation is:
Metal + Acid → Salt + Hydrogen Gas
For example, the reaction between zinc and hydrochloric acid is:
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Here, zinc displaces hydrogen from hydrochloric acid, forming zinc chloride and hydrogen gas. The hydrogen gas produced can be observed as bubbles.
Factors Affecting the Reaction with Acids:
- Concentration of the acid: Higher acid concentration leads to a faster reaction rate.
- Temperature: Increasing temperature increases the kinetic energy of the reactants, leading to more frequent and energetic collisions, resulting in a faster reaction.
- Surface area of the metal: A larger surface area (e.g., using powdered metal instead of a solid block) increases the contact between the metal and the acid, accelerating the reaction.
- Nature of the acid: Strong acids (like hydrochloric acid, sulfuric acid, and nitric acid) react more vigorously than weak acids (like acetic acid). Note that nitric acid's reactivity is complex, and often doesn't simply produce hydrogen gas.
- Presence of impurities: Impurities on the metal's surface can either catalyze or inhibit the reaction.
Specific Examples of Metal-Acid Reactions:
- Alkali metals with water (aqueous acid): These metals react violently with even dilute acids, often producing a flame due to the highly exothermic nature of the reaction.
- Iron with sulfuric acid: This reaction is used industrially to produce iron(II) sulfate.
- Magnesium with hydrochloric acid: A common demonstration in chemistry classes, this reaction produces hydrogen gas readily.
Reactions of Metals with Bases
The reaction of metals with bases is less common than with acids and is generally limited to certain metals, specifically those that are amphoteric. Amphoteric metals can react with both acids and bases. Aluminum and zinc are prime examples. These reactions typically involve the formation of a complex ion and hydrogen gas.
The reaction is generally expressed as:
Metal + Base + Water → Metal Salt + Hydrogen Gas
For instance, the reaction of aluminum with sodium hydroxide is:
2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g)
In this reaction, aluminum reacts with sodium hydroxide and water to produce sodium tetrahydroxoaluminate(III) and hydrogen gas. Note the formation of a complex ion, [Al(OH)₄]⁻.
Factors Affecting the Reaction with Bases:
- Concentration of the base: Similar to acid reactions, a higher base concentration generally increases the reaction rate.
- Temperature: Higher temperatures increase the reaction rate.
- Surface area of the metal: A larger surface area increases the reaction rate.
- Nature of the base: Stronger bases tend to react more vigorously.
Specific Examples of Metal-Base Reactions:
- Aluminum with sodium hydroxide: This reaction is used in the industrial production of aluminum compounds.
- Zinc with potassium hydroxide: Similar to the aluminum reaction, this produces a complex zincate ion and hydrogen gas.
Exceptions and Complexities
The descriptions above represent the general trends. However, several exceptions and complexities exist:
-
Passivation: Some metals, like aluminum and chromium, form a protective oxide layer on their surface when exposed to air or certain solutions. This oxide layer prevents further reaction with acids or bases, a phenomenon called passivation. This is why aluminum cookware doesn't readily react with most foods.
-
Nitric Acid: Nitric acid is a strong oxidizing agent and its reactions with metals are often more complex than simple displacement reactions. Instead of hydrogen gas, nitrogen oxides are frequently produced. The metal's reactivity and the concentration of the nitric acid significantly influence the reaction products.
-
Concentrated Sulfuric Acid: Concentrated sulfuric acid is also a strong oxidizing agent. It can react with metals in a way that produces sulfur dioxide rather than hydrogen.
-
Presence of other ions: The presence of other ions in the solution can influence the reaction, possibly altering the products formed or the reaction rate.
Applications and Industrial Significance
The reactions between metals, acids, and bases have numerous applications in various industries:
- Metal Refining: Acid leaching is used to extract metals from their ores.
- Production of Metal Salts: The reactions are used extensively in the chemical industry to produce various metal salts.
- Cleaning Metals: Acids are used to clean metal surfaces, removing oxides and other impurities.
- Etching: Acids are used in etching processes to create patterns on metal surfaces.
- Battery Production: Many batteries utilize the reactions between metals and acids or bases to generate electricity.
Conclusion
The reactivity of metals with acids and bases is a complex subject with many variables influencing the outcome. While the general principles outlined above provide a framework for understanding these reactions, specific cases can exhibit unique behavior due to factors such as the metal's position in the reactivity series, the strength and concentration of the acid or base, temperature, surface area, and the presence of other substances. Understanding these complexities is essential for anyone working with metals and chemical reactions in various industrial and research settings. Further exploration into specific metal-acid and metal-base reactions will reveal even more intricacies in this fascinating area of chemistry.
Latest Posts
Latest Posts
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Or Bases React With Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
