Covalent Bonds Are Formed Between Two Non-metals
Muz Play
Mar 28, 2025 · 7 min read
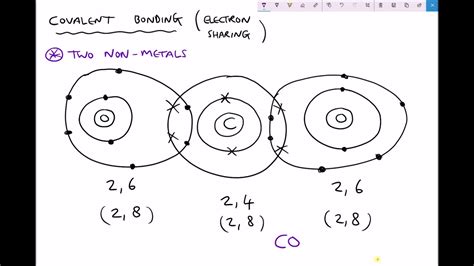
Table of Contents
Covalent Bonds: A Deep Dive into Non-Metal Bonding
Covalent bonds are a fundamental concept in chemistry, representing the cornerstone of countless molecules essential to life and modern materials. Understanding how and why these bonds form, particularly between two non-metal atoms, is crucial for grasping the properties and behaviors of a vast array of substances. This comprehensive article will delve into the intricacies of covalent bonding, exploring its mechanisms, characteristics, and the diverse range of molecules it creates.
What are Covalent Bonds?
A covalent bond is a chemical bond formed when two atoms share one or more pairs of electrons. Unlike ionic bonds, which involve the transfer of electrons from one atom to another, covalent bonds result from a mutual attraction between atoms that are seeking to achieve a stable electron configuration, usually a full outermost electron shell (octet rule). This sharing allows both atoms to effectively "fill" their valence shells and achieve greater stability. This sharing creates a strong attractive force holding the atoms together, forming a molecule.
The Role of Non-Metals
The formation of covalent bonds is particularly prevalent between non-metal atoms. Non-metals typically have high electronegativity, meaning they have a strong attraction for electrons. When two non-metal atoms approach each other, neither atom readily gives up its electrons to the other. Instead, they achieve stability by sharing electrons, creating a covalent bond. This contrasts sharply with ionic bonding, where a metal atom (low electronegativity) readily donates electrons to a non-metal atom (high electronegativity).
Mechanisms of Covalent Bond Formation
The formation of a covalent bond can be explained using several key concepts:
1. Valence Electrons: The Key Players
The outermost electrons of an atom, known as valence electrons, are the primary participants in covalent bond formation. The number of valence electrons determines how many bonds an atom can form. For example, carbon, with four valence electrons, can form four covalent bonds. Oxygen, with six valence electrons, typically forms two covalent bonds.
2. Octet Rule: Achieving Stability
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable electron configuration with eight electrons in their outermost shell. This configuration is particularly stable due to the filled s and p orbitals. However, there are exceptions to the octet rule, particularly for elements in periods beyond the second (e.g., phosphorus, sulfur).
3. Electron Sharing: The Bond Formation
When two non-metal atoms approach each other, their valence electrons interact. If the energy is favorable (lowering the overall energy of the system), a pair of electrons is shared between the two atoms. This shared pair of electrons constitutes the covalent bond. The shared electrons are attracted to the positively charged nuclei of both atoms, holding them together.
4. Overlapping Orbitals: The Quantum Mechanical Perspective
A more precise explanation involves the overlapping of atomic orbitals. When orbitals from different atoms overlap, a region of high electron density is created between the nuclei. This region represents the covalent bond, and the electrons residing in this region are shared between the two atoms. The greater the overlap, the stronger the bond.
Types of Covalent Bonds
Covalent bonds aren't all created equal; they exhibit variations based on the number of electron pairs shared:
1. Single Bonds: Sharing One Pair
A single bond involves the sharing of one pair of electrons between two atoms. This is represented by a single line (-) in a structural formula. For example, the bond in H₂ (hydrogen gas) is a single covalent bond.
2. Double Bonds: Sharing Two Pairs
A double bond involves the sharing of two pairs of electrons. This is represented by two lines (=) in a structural formula. A classic example is the carbon-oxygen double bond in carbon dioxide (CO₂). Double bonds are generally shorter and stronger than single bonds.
3. Triple Bonds: Sharing Three Pairs
A triple bond involves the sharing of three pairs of electrons. This is represented by three lines (≡) in a structural formula. Nitrogen gas (N₂) is a prime example, with a strong triple bond between the two nitrogen atoms. Triple bonds are the shortest and strongest type of covalent bond.
Properties of Covalently Bonded Substances
Substances formed through covalent bonds exhibit distinct properties:
-
Lower melting and boiling points: Compared to ionic compounds, covalent compounds generally have lower melting and boiling points because the intermolecular forces (forces between molecules) are weaker than the strong electrostatic forces in ionic compounds.
-
Poor electrical conductivity: Covalent compounds typically do not conduct electricity in either solid or liquid states because they do not have freely moving ions or electrons.
-
Solubility variations: Solubility in water varies widely depending on the polarity of the molecule. Polar covalent molecules (those with unequal sharing of electrons) tend to be soluble in water, while nonpolar molecules are often insoluble.
-
Often exist as gases, liquids, or low-melting solids: The weaker intermolecular forces lead to lower melting and boiling points, resulting in many covalent compounds being gases or liquids at room temperature.
Examples of Covalent Compounds
Covalent bonds are ubiquitous in chemistry, forming the basis of countless molecules crucial to life and technology:
-
Water (H₂O): A polar covalent molecule essential for life, exhibiting strong hydrogen bonding.
-
Carbon Dioxide (CO₂): A nonpolar covalent molecule involved in the carbon cycle and climate change.
-
Methane (CH₄): A nonpolar covalent molecule, the primary component of natural gas.
-
Glucose (C₆H₁₂O₆): A complex sugar molecule composed of multiple covalent bonds, providing energy to living organisms.
-
Proteins: Polymers of amino acids held together by peptide bonds (a type of covalent bond).
-
DNA and RNA: Nucleic acids built from nucleotides linked by covalent phosphodiester bonds.
-
Polymers: Many synthetic polymers, like plastics and rubbers, are made of long chains of covalently bonded atoms.
Polarity in Covalent Bonds
Not all covalent bonds are created equal in terms of electron sharing. The concept of electronegativity plays a crucial role in determining the polarity of a covalent bond:
Electronegativity and Polarity
Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. When two atoms with different electronegativities form a covalent bond, the electrons are not shared equally. The atom with the higher electronegativity attracts the electrons more strongly, creating a polar covalent bond. This results in a partial negative charge (δ-) on the more electronegative atom and a partial positive charge (δ+) on the less electronegative atom.
Nonpolar Covalent Bonds
If two atoms have similar electronegativities, the electrons are shared almost equally, resulting in a nonpolar covalent bond. In this case, there is no significant charge separation.
Consequences of Polarity
The polarity of a covalent bond significantly influences the properties of the molecule. Polar molecules often have higher boiling points and are more soluble in polar solvents like water compared to nonpolar molecules.
Exceptions to the Octet Rule
While the octet rule provides a useful framework for understanding covalent bonding, there are important exceptions:
-
Electron-deficient molecules: Some molecules, like boron trifluoride (BF₃), have fewer than eight valence electrons around the central atom.
-
Expanded octets: Elements in the third period and beyond can accommodate more than eight valence electrons, as their d orbitals can participate in bonding. Examples include sulfur hexafluoride (SF₆) and phosphorus pentachloride (PCl₅).
-
Odd-electron molecules: Some molecules have an odd number of valence electrons, making it impossible for all atoms to achieve an octet. Nitric oxide (NO) is a classic example.
Resonance Structures
In some molecules, the bonding electrons can be delocalized over multiple atoms, represented by resonance structures. This means that the actual structure is a hybrid of several possible Lewis structures. Benzene (C₆H₆) is a prime example, with delocalized electrons creating a particularly stable ring structure.
Hydrogen Bonding: A Special Type of Intermolecular Force
Hydrogen bonding is a particularly strong type of intermolecular force (forces between molecules) that occurs when a hydrogen atom covalently bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This type of bonding is crucial in determining the properties of water and many biological molecules.
Conclusion
Covalent bonds are fundamental to the structure and function of countless molecules, from simple diatomic gases to complex biological macromolecules. Understanding the principles of covalent bond formation, the different types of covalent bonds, and the influence of electronegativity and other factors is essential for comprehending the behavior and properties of a wide range of chemical substances. This knowledge is not only crucial for fundamental chemistry but also finds applications in diverse fields like materials science, biochemistry, and nanotechnology. The exploration of covalent bonds continues to be a vibrant area of research, constantly revealing new insights into the intricate world of molecular interactions.
Latest Posts
Latest Posts
-
What Happens When You Combine An Acid And A Base
Mar 31, 2025
-
How To Solve A Rational Exponent
Mar 31, 2025
-
Heat Of Combustion Of Benzoic Acid
Mar 31, 2025
-
How To Round Sig Figs When Adding
Mar 31, 2025
-
Do Acids Or Bases React With Metals
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Covalent Bonds Are Formed Between Two Non-metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
