Heat Of Combustion Of Benzoic Acid
Muz Play
Mar 31, 2025 · 6 min read
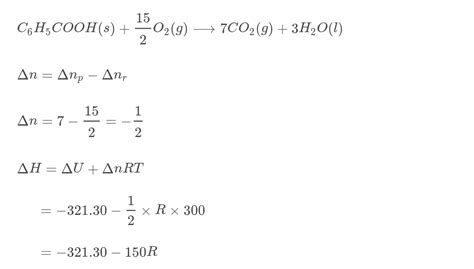
Table of Contents
The Heat of Combustion of Benzoic Acid: A Comprehensive Guide
Benzoic acid, a simple aromatic carboxylic acid with the formula C₇H₆O₂, holds a significant place in calorimetry, the science of measuring heat. Its widespread use as a standard for calibrating calorimeters stems from its readily available high purity, ease of handling, and well-established heat of combustion. This article delves into the intricacies of benzoic acid's heat of combustion, exploring its determination, applications, and the factors influencing its value.
Understanding Heat of Combustion
The heat of combustion, also known as the enthalpy of combustion, represents the heat released when one mole of a substance undergoes complete combustion in excess oxygen under standard conditions (typically 298.15 K and 1 atm). For benzoic acid, this combustion reaction is represented as:
C₇H₆O₂(s) + 15/2 O₂(g) → 7CO₂(g) + 3H₂O(l)
The heat released during this reaction is exothermic, meaning it's a negative value. The standard molar enthalpy of combustion (ΔHc°) for benzoic acid is precisely determined and serves as a crucial reference point in calorimetry.
Why Benzoic Acid is the Gold Standard
Several factors contribute to benzoic acid's status as the primary standard in bomb calorimetry:
-
High Purity: Benzoic acid is readily available in high purity (99.9% or greater), minimizing uncertainties in measurements. Impurities can significantly affect the heat of combustion.
-
Well-Defined Heat of Combustion: Its heat of combustion has been meticulously determined through numerous experiments, resulting in a widely accepted and reliable value. This allows for accurate calibration of calorimeters.
-
Ease of Handling: It's a solid at room temperature, relatively non-hygroscopic (doesn't readily absorb moisture from the air), and easy to handle and weigh accurately.
-
Complete Combustion: Benzoic acid undergoes complete combustion readily, ensuring consistent and reliable results. Incomplete combustion would lead to inaccurate measurements.
-
Stable and Non-Volatile: Its stability and low volatility ensure that mass loss during handling and combustion is negligible, further enhancing measurement accuracy.
Determining the Heat of Combustion of Benzoic Acid
The heat of combustion of benzoic acid is typically determined using a bomb calorimeter, a device designed to measure the heat released during a combustion reaction at constant volume. The process involves the following steps:
-
Sample Preparation: A precisely weighed amount of benzoic acid is pressed into a pellet to ensure uniform combustion.
-
Bomb Assembly: The benzoic acid pellet is placed in the bomb, a sealed, strong metal container. A small amount of oxygen is introduced into the bomb to facilitate complete combustion.
-
Immersion in Water: The bomb is immersed in a known mass of water within the calorimeter. A thermometer measures the temperature change of the water.
-
Ignition: The benzoic acid is ignited electrically, initiating the combustion reaction.
-
Temperature Measurement: The temperature rise of the water is precisely monitored. This temperature change is directly related to the heat released during the combustion reaction.
-
Calibration: The calorimeter's heat capacity (the amount of heat required to raise the temperature of the calorimeter by 1°C) must be determined using a known substance with a well-established heat of combustion, such as benzoic acid.
-
Calculation: The heat of combustion of the benzoic acid sample is calculated using the following equation:
ΔHc = - (q_water + q_calorimeter) / n
Where:
- ΔHc is the heat of combustion
- q_water is the heat absorbed by the water (mass of water × specific heat of water × temperature change)
- q_calorimeter is the heat absorbed by the calorimeter (heat capacity of calorimeter × temperature change)
- n is the number of moles of benzoic acid
The accepted value for the standard molar enthalpy of combustion of benzoic acid is approximately -3227 kJ/mol. However, this value can slightly vary depending on the purity of the sample and the experimental conditions.
Factors Affecting the Heat of Combustion
Several factors can influence the measured heat of combustion of benzoic acid:
-
Purity of Benzoic Acid: Impurities in the sample can significantly affect the measured heat of combustion, leading to inaccurate results. High-purity benzoic acid is crucial for accurate measurements.
-
Oxygen Pressure: Insufficient oxygen can lead to incomplete combustion, resulting in a lower measured heat of combustion. Sufficient oxygen pressure is essential to ensure complete combustion.
-
Calorimeter Calibration: An inaccurately calibrated calorimeter can lead to errors in the measured heat of combustion. Proper calibration is crucial for accurate measurements.
-
Temperature Measurement: Accurate temperature measurements are vital for determining the heat released during the combustion reaction. Precise thermometry is necessary.
-
Heat Loss: Heat loss to the surroundings can affect the accuracy of the measurements. Proper insulation of the calorimeter minimizes heat loss.
Applications of Benzoic Acid's Heat of Combustion
The precisely known heat of combustion of benzoic acid has numerous applications in various fields:
-
Calorimeter Calibration: As mentioned earlier, it's the primary standard for calibrating bomb calorimeters used to determine the heat of combustion of other substances. This calibration is crucial for accurate measurements in diverse research areas.
-
Determining the Heat of Combustion of Other Substances: Once the calorimeter is calibrated using benzoic acid, it can be used to measure the heat of combustion of other organic compounds, aiding in the study of reaction thermodynamics and energy content of fuels.
-
Food and Nutrition: The heat of combustion is crucial in determining the caloric content of food, providing essential information for nutrition labeling and dietary planning. While not directly used for food analysis, benzoic acid plays a key role in calorimeter calibration.
-
Industrial Applications: In industrial processes, determining the energy content of fuels is crucial for efficient combustion and process optimization. Benzoic acid assists in calibrating the instruments used for these measurements.
-
Environmental Studies: Understanding the heat of combustion is vital in studies related to energy efficiency, waste management, and environmental impact assessments. Accurate measurement of heat release is crucial for these applications.
Conclusion
The heat of combustion of benzoic acid is a fundamental parameter in calorimetry, serving as a cornerstone for accurate and reliable measurements of energy changes in chemical reactions. Its readily available high purity, well-defined heat of combustion, and ease of handling make it the ideal standard for calibrating calorimeters. The precise determination of this value has wide-ranging applications in various fields, from fundamental research to industrial processes and environmental studies. Understanding the factors that can influence the measurement of benzoic acid's heat of combustion is crucial for ensuring accuracy and reliability in calorimetric experiments. The continued use of benzoic acid as a standard underscores its importance in the advancement of calorimetric techniques and their application to a vast array of scientific and industrial endeavors. Future research may explore alternative standards, but the legacy and importance of benzoic acid in calorimetry will undoubtedly remain significant.
Latest Posts
Latest Posts
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
-
What Does A Negative Reduction Potential Mean
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Heat Of Combustion Of Benzoic Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
