What Happens When You Combine An Acid And A Base
Muz Play
Mar 31, 2025 · 6 min read
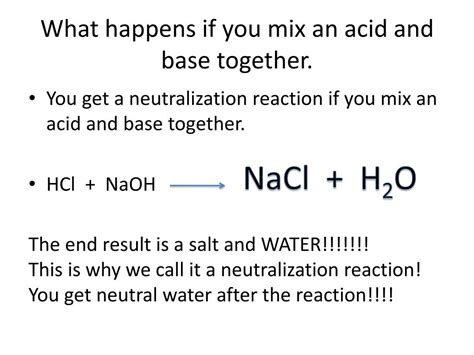
Table of Contents
What Happens When You Combine an Acid and a Base? A Deep Dive into Neutralization Reactions
The seemingly simple act of mixing an acid and a base triggers a fundamental chemical reaction with far-reaching consequences, impacting everything from our daily lives to industrial processes. This reaction, known as neutralization, is a cornerstone of chemistry, playing a crucial role in various fields, from environmental science to medicine. Understanding the intricacies of neutralization is key to grasping the broader principles of acid-base chemistry.
Understanding Acids and Bases: A Quick Refresher
Before delving into the specifics of neutralization, let's establish a clear understanding of acids and bases. While several definitions exist (Arrhenius, Brønsted-Lowry, Lewis), we'll focus on the Brønsted-Lowry definition for its broad applicability.
Brønsted-Lowry Definition:
-
Acids: Acids are substances that donate a proton (H⁺ ion). Think of them as proton donors. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and acetic acid (CH₃COOH). Strong acids readily donate their protons, while weak acids donate them less readily.
-
Bases: Bases are substances that accept a proton (H⁺ ion). They are proton acceptors. Common examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH₃). Similar to acids, we have strong and weak bases, differentiated by their proton-accepting ability.
The Neutralization Reaction: The Heart of the Matter
When an acid and a base are combined, a neutralization reaction occurs. This reaction involves the transfer of protons from the acid to the base, resulting in the formation of water and a salt.
The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
Let's break this down:
-
Acid: Provides the H⁺ ions.
-
Base: Provides the OH⁻ ions (hydroxide ions).
-
Salt: An ionic compound formed from the cation of the base and the anion of the acid. The properties of the salt will depend on the specific acid and base involved.
-
Water: Formed from the combination of H⁺ ions from the acid and OH⁻ ions from the base. This is why the resulting solution is often less acidic or basic than the original components.
A Closer Look at the Reaction:
The neutralization reaction is essentially a proton transfer process. The H⁺ ion from the acid combines with the OH⁻ ion from the base to form a water molecule (H₂O). This leaves behind the cation from the base and the anion from the acid, which combine to form the salt.
Example 1: Neutralization of Hydrochloric Acid (HCl) with Sodium Hydroxide (NaOH)
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
In this reaction:
- HCl donates a proton (H⁺).
- NaOH accepts the proton.
- NaCl (sodium chloride, or common table salt) is formed.
- Water (H₂O) is formed.
Example 2: Neutralization of Sulfuric Acid (H₂SO₄) with Potassium Hydroxide (KOH)
H₂SO₄ (aq) + 2KOH (aq) → K₂SO₄ (aq) + 2H₂O (l)
Note that in this reaction, two moles of KOH are required to neutralize one mole of H₂SO₄ because sulfuric acid is a diprotic acid (donates two protons).
The pH Scale and Neutralization
The pH scale measures the acidity or alkalinity of a solution. A pH of 7 is considered neutral, values below 7 are acidic, and values above 7 are alkaline (basic). Neutralization reactions lead to a change in pH, typically moving towards a neutral pH of 7.
The extent of the pH change depends on several factors, including:
-
Strength of the acid and base: Strong acids and strong bases undergo complete neutralization, resulting in a more dramatic pH shift. Weak acids and weak bases may not undergo complete neutralization, leading to a less pronounced pH change.
-
Concentration of the acid and base: Higher concentrations result in a greater pH change.
-
Volume of the acid and base: The relative volumes of the acid and base influence the final pH.
Beyond the Basics: Applications of Neutralization
Neutralization reactions are not just a theoretical concept; they have widespread practical applications across various domains:
1. Industrial Processes:
-
Wastewater Treatment: Neutralization is crucial in treating industrial wastewater containing acidic or basic pollutants. Adding a base to acidic wastewater or an acid to basic wastewater neutralizes the pH, making it safer for disposal.
-
Chemical Synthesis: Many chemical syntheses rely on precise pH control, often achieved through neutralization reactions.
-
Food Production: Neutralization plays a role in food processing, for instance, in adjusting the pH of certain ingredients.
2. Environmental Applications:
-
Acid Rain Mitigation: Acid rain, caused by the release of acidic gases into the atmosphere, can damage ecosystems. Neutralizing agents can be used to mitigate the effects of acid rain on soil and water bodies.
-
Soil Remediation: Neutralization is employed to adjust the pH of soil, making it suitable for specific plant growth. Acidity or alkalinity in soil can hinder plant growth.
3. Biological Systems:
-
Human Body: The human body employs buffer systems to maintain a relatively constant pH. These buffer systems are essentially neutralization reactions that prevent drastic pH changes. Maintaining a stable pH is crucial for many biological processes.
-
Digestion: The digestive system uses acids (like hydrochloric acid in the stomach) and bases to aid digestion. Neutralization processes are involved in maintaining the optimal pH in various parts of the digestive tract.
4. Everyday Life:
-
Antacid Medications: Antacids, used to relieve heartburn and indigestion, are bases that neutralize excess stomach acid.
-
Cleaning Products: Many cleaning products utilize acids or bases to remove stains or disinfect surfaces. The neutralization reaction may be part of the cleaning process.
Titration: Quantifying Neutralization
Titration is a laboratory technique used to determine the concentration of an unknown acid or base by reacting it with a solution of known concentration. This process involves carefully adding a solution of known concentration (the titrant) to a solution of unknown concentration until the reaction is complete, often signaled by a color change using an indicator.
Titration provides a quantitative measure of the neutralization reaction, allowing precise determination of the unknown concentration. This technique is vital in various analytical chemistry applications, including environmental monitoring and quality control in industrial processes.
Safety Precautions: Handling Acids and Bases
Acids and bases can be corrosive and potentially hazardous. When handling these substances, it's crucial to take the following safety precautions:
-
Wear appropriate safety gear: This includes safety goggles, gloves, and a lab coat.
-
Work in a well-ventilated area: Some acids and bases release harmful fumes.
-
Handle acids and bases carefully: Avoid spills and direct contact with skin or eyes.
-
Neutralize spills appropriately: Consult safety data sheets (SDS) for specific instructions on neutralizing spills.
Conclusion: The Significance of Neutralization
The neutralization reaction is a fundamental chemical process with far-reaching implications. Understanding the principles behind this reaction is key to comprehending a wide range of scientific phenomena, from industrial processes to biological systems. Its importance in various applications highlights its crucial role in chemistry and beyond, emphasizing the need for safe handling and responsible use of acids and bases. The versatility and significance of neutralization reactions reinforce its status as a core concept in chemistry and a cornerstone of many practical applications. From environmental remediation to everyday life, the impact of acid-base neutralization is undeniable.
Latest Posts
Latest Posts
-
Effective Nuclear Charge Periodic Table Trend
Apr 01, 2025
-
Examples Of Instantaneous Rate Of Change
Apr 01, 2025
-
How Is The Use Of Symbols Related To Culture
Apr 01, 2025
-
As You Move Across The Periodic Table
Apr 01, 2025
-
What Is In The Atmosphere Of Jupiter
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Happens When You Combine An Acid And A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
