Effective Nuclear Charge Periodic Table Trend
Muz Play
Apr 01, 2025 · 6 min read
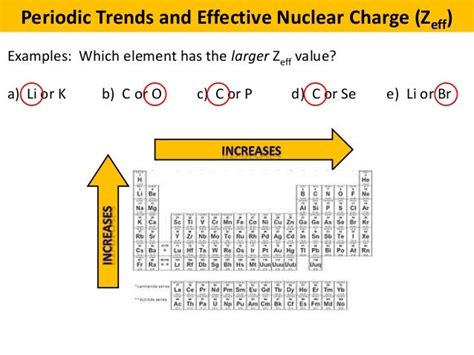
Table of Contents
Effective Nuclear Charge: A Periodic Trend Unveiling Atomic Behavior
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and recurring properties. Understanding these properties is crucial, and one fundamental concept driving them is the effective nuclear charge (Z<sub>eff</sub>). This article delves deep into the effective nuclear charge, exploring its definition, how it's calculated, its periodic trends, and its profound impact on various atomic properties. We’ll unravel its influence on atomic radius, ionization energy, and electronegativity, showcasing its central role in understanding chemical behavior.
Understanding Effective Nuclear Charge (Z<sub>eff</sub>)
At the heart of an atom lies the nucleus, containing positively charged protons and neutral neutrons. Surrounding the nucleus are negatively charged electrons, arranged in shells and subshells. The effective nuclear charge represents the net positive charge experienced by an electron in a many-electron atom. It's not simply the total number of protons (atomic number, Z), because the negatively charged electrons shield each other from the full attractive force of the nucleus. This shielding effect significantly reduces the positive charge felt by each electron.
In simpler terms: Imagine the nucleus as a powerful magnet and electrons as smaller magnets repelling each other. The outermost electrons don't experience the full pull of the nucleus; some of its force is neutralized by the inner electrons acting as a shield. Z<sub>eff</sub> quantifies the net attractive force after considering this shielding.
Calculating Effective Nuclear Charge: Slater's Rules
Precise calculation of Z<sub>eff</sub> for multi-electron atoms requires complex quantum mechanical methods. However, a simpler, albeit approximate, method exists: Slater's rules. These rules provide a relatively straightforward way to estimate Z<sub>eff</sub> for each electron in an atom.
Slater's rules involve assigning shielding constants (S) to each electron based on its position within the electron configuration. The effective nuclear charge is then calculated as:
Z<sub>eff</sub> = Z - S
Where:
- Z is the atomic number (number of protons)
- S is the shielding constant
The calculation of S follows specific rules, varying based on the electron's shell and subshell. For example, electrons in the same shell generally provide less shielding than electrons in inner shells. Different coefficients are applied to electrons within the same principal quantum number (n) and those in lower principal quantum numbers. While Slater's rules provide an approximation, they offer valuable insight into the relative shielding experienced by electrons in different atomic orbitals.
Periodic Trends in Effective Nuclear Charge
The effective nuclear charge isn't uniform across the periodic table. It exhibits distinct trends that directly influence the chemical properties of elements.
Across a Period (Left to Right):
As we move across a period from left to right, the atomic number (Z) increases by one with each element. Electrons are added to the same principal energy level (shell). Although the shielding effect increases slightly due to electron-electron repulsion, the increase in nuclear charge (Z) significantly outweighs the increase in shielding. Therefore, Z<sub>eff</sub> increases steadily across a period.
This increase in Z<sub>eff</sub> leads to a stronger attraction between the nucleus and the outermost electrons, resulting in a decrease in atomic radius and an increase in ionization energy and electronegativity (explained in detail later).
Down a Group (Top to Bottom):
Moving down a group, both the atomic number (Z) and the number of electron shells increase. The added electrons fill new, higher energy levels, effectively shielding the outer electrons from the increased nuclear charge. While Z increases, the increase in shielding is even more significant. Consequently, Z<sub>eff</sub> increases only slightly, or even remains relatively constant, down a group.
This relatively small change in Z<sub>eff</sub> down a group leads to an increase in atomic radius and a decrease in ionization energy and electronegativity, reflecting the weakening attraction between the nucleus and the outermost electrons.
Impact of Effective Nuclear Charge on Atomic Properties
The effective nuclear charge is not just a theoretical concept; it has a direct and measurable influence on several key atomic properties:
1. Atomic Radius:
Atomic radius, the distance from the nucleus to the outermost electron, is inversely proportional to the effective nuclear charge. A higher Z<sub>eff</sub> results in a smaller atomic radius because the stronger pull of the nucleus draws the electrons closer. This explains the decreasing atomic radius trend across a period and the increasing trend down a group.
2. Ionization Energy:
Ionization energy is the energy required to remove an electron from an atom. A higher Z<sub>eff</sub> leads to a higher ionization energy. The stronger the attraction between the nucleus and the electron, the more energy is required to overcome this attraction and remove the electron. This explains the general increase in ionization energy across a period and the decrease down a group.
3. Electronegativity:
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Higher Z<sub>eff</sub> corresponds to higher electronegativity. Atoms with a higher effective nuclear charge can more effectively attract shared electrons in a covalent bond. Hence, electronegativity generally increases across a period and decreases down a group, mirroring the trend in Z<sub>eff</sub>.
4. Metallic Character:
Effective nuclear charge plays a crucial role in determining metallic character. Elements with low Z<sub>eff</sub> generally exhibit strong metallic character. This is because the outer electrons are loosely held and can easily be delocalized, contributing to metallic bonding. Conversely, elements with high Z<sub>eff</sub> tend to have weaker metallic character, as their outer electrons are strongly held by the nucleus.
Exceptions and Refinements
While the trends discussed above generally hold true, exceptions exist. These exceptions often arise due to electron configurations and inter-electron repulsions, which can subtly modify the effective nuclear charge experienced by electrons.
For instance, the slight dip in ionization energy between groups 2 and 13 is attributed to the increased shielding offered by the filled s subshell, leading to a slight reduction in Z<sub>eff</sub> for the p electrons in group 13. Similar subtle variations occur throughout the periodic table, reminding us that Z<sub>eff</sub> is an approximation, though a powerful one for understanding overall periodic trends.
Conclusion: Z<sub>eff</sub> – A Key to Understanding Chemical Behavior
The effective nuclear charge is a fundamental concept explaining the periodic trends in atomic properties. By considering the shielding effect of inner electrons on the outer electrons, Z<sub>eff</sub> provides a quantitative measure of the net attractive force experienced by electrons in an atom. This, in turn, directly influences atomic radius, ionization energy, electronegativity, and other critical properties that govern the chemical behavior of elements. While approximate calculations like Slater's rules provide valuable insight, the underlying principle remains: the stronger the effective nuclear charge, the tighter the electrons are held, influencing the chemical reactivity and bonding characteristics of the element. Understanding Z<sub>eff</sub> is therefore essential for a deeper appreciation of the periodic table and the fascinating world of chemical bonding and reactivity.
Latest Posts
Latest Posts
-
The Final Electron Acceptor Of Cellular Respiration Is
Apr 02, 2025
-
Monomers Are Connected In What Type Of Reaction
Apr 02, 2025
-
Find The Equation Of The Vertical Line
Apr 02, 2025
-
Electron Configuration For Copper And Chromium
Apr 02, 2025
-
Confidence Interval Calculator With Two Samples
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Effective Nuclear Charge Periodic Table Trend . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
