How Does Auger Electron Spectroscopy Work
Muz Play
Mar 22, 2025 · 7 min read
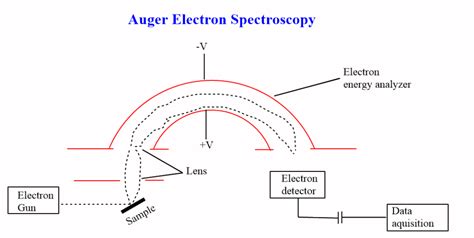
Table of Contents
How Does Auger Electron Spectroscopy Work? A Deep Dive into Surface Analysis
Auger electron spectroscopy (AES) is a powerful surface-sensitive analytical technique used to determine the elemental composition of a material's surface. It's particularly valuable for studying the top few atomic layers (typically the top 1-10 nm), providing crucial insights into surface phenomena relevant to diverse fields like materials science, chemistry, and nanotechnology. Understanding how AES works requires a grasp of fundamental physics and the intricacies of electron interactions within a material. This article provides a comprehensive explanation of the underlying principles, instrumentation, data analysis, and applications of AES.
The Core Principle: Auger Electron Emission
At the heart of AES lies the Auger effect, a radiationless process involving the ejection of an electron from an atom. This process is initiated by the interaction of a high-energy primary electron beam (typically 3-20 keV) with the sample. Let's break down the sequence of events:
1. Core-level Ionization
The incident electron beam possesses sufficient energy to knock out a core-level electron from an atom in the sample. This leaves a core-level hole, creating an unstable atomic configuration. The energy required to remove this core-level electron is characteristic of the element and its chemical state.
2. Relaxation through Auger Emission
The atom is now in a highly energetic, unstable state. To regain stability, an electron from a higher energy level (e.g., a valence or less tightly bound core level) will transition down to fill the core-level hole. This transition releases energy.
3. Auger Electron Ejection
Instead of emitting a photon (as in X-ray fluorescence), the energy released during the relaxation process can be transferred to another electron (the Auger electron) in a higher energy level. This Auger electron is then ejected from the atom with a kinetic energy characteristic of the element and its chemical state.
4. Energy Measurement and Elemental Identification
The ejected Auger electrons are then collected and energy-analyzed using an electron energy analyzer. The kinetic energy of the Auger electrons is precisely determined, and this energy is directly related to the element from which it originated. A spectrum is generated plotting the intensity of Auger electrons against their kinetic energies. The spectrum reveals the presence and concentration of elements within the probed surface region. The peaks in the spectrum correspond to specific Auger transitions, acting as fingerprints for individual elements.
Instrumentation: Key Components of an AES System
A typical Auger electron spectrometer consists of several crucial components:
1. Electron Gun: The Source of Excitation
The electron gun generates a finely focused beam of high-energy electrons directed at the sample's surface. The gun parameters (beam energy, current, and spot size) can be controlled to optimize the analysis for specific applications. A smaller spot size enhances spatial resolution, allowing for analysis of very small areas.
2. Ultra-High Vacuum (UHV) Chamber: Maintaining Surface Integrity
AES requires an ultra-high vacuum environment (typically 10<sup>-8</sup> to 10<sup>-10</sup> Pa) to minimize surface contamination. Contamination from residual gases can significantly alter the surface composition and obstruct accurate analysis. The UHV chamber maintains a clean environment, preventing significant interaction between the sample and residual gases.
3. Electron Energy Analyzer: Sorting the Electrons by Energy
This crucial component is responsible for precisely measuring the kinetic energy of the emitted Auger electrons. Common analyzer types include cylindrical mirror analyzers (CMAs) and hemispherical analyzers. CMAs offer high sensitivity and collection efficiency, while hemispherical analyzers are preferred for higher energy resolution.
4. Detection System: Counting the Auger Electrons
The analyzer directs the electrons with specific energies towards a detection system, typically a channel electron multiplier (CEM) or a multichannel plate (MCP). These detectors amplify the weak Auger electron signal, allowing for accurate quantification of the element concentrations.
5. Sample Manipulation System: Positioning and Treating the Sample
Sophisticated sample manipulation systems are essential for precise positioning and possible in-situ treatment (e.g., heating, cooling, ion sputtering) of the sample during analysis. This ensures optimal positioning of the sample for analysis and allows for depth profiling.
6. Data Acquisition and Processing System: Interpreting the Results
The data acquisition system collects the signals from the detector and processes them to create an Auger spectrum. Specialized software is employed for data processing, background subtraction, peak fitting, and quantitative analysis.
Data Analysis: Interpreting the Auger Spectrum
The raw data obtained from AES is a spectrum showing the intensity of detected electrons as a function of their kinetic energy. However, extracting meaningful information from this spectrum requires careful data analysis. Key steps include:
1. Background Subtraction: Removing Noise
The raw spectrum often contains a significant background signal arising from inelastically scattered electrons. This background needs to be subtracted to isolate the Auger peaks. Various background subtraction techniques are used, such as Shirley background subtraction or linear background subtraction.
2. Peak Identification and Quantification: Assigning Elements
The distinct Auger peaks are identified by comparing their energy with known Auger transition energies of different elements. Quantitative analysis involves determining the elemental concentrations based on the peak intensities. This usually involves using sensitivity factors specific to the Auger transitions and the instrument settings.
3. Depth Profiling: Revealing Layered Structures
By combining AES with ion sputtering (e.g., Ar<sup>+</sup> sputtering), depth profiles can be obtained. Ion sputtering gradually removes surface layers, allowing the sequential analysis of the underlying layers. This reveals the elemental composition as a function of depth, crucial for studying layered structures or thin films.
Applications: Where AES Excels
AES finds applications in a wide range of scientific and industrial fields, including:
- Materials Science: Characterizing the surface composition of metals, alloys, semiconductors, and ceramics. Identifying surface contaminants and understanding surface oxidation processes.
- Semiconductor Industry: Analyzing the cleanliness and composition of semiconductor wafers, identifying contaminants that affect device performance. Studying the interfaces in layered semiconductor structures.
- Corrosion Science: Investigating the formation and growth of corrosion layers, identifying the elemental composition of corrosion products.
- Catalysis: Studying the surface composition of catalysts, understanding how the surface structure and composition influence catalytic activity.
- Nanotechnology: Characterizing the composition and structure of nanomaterials, probing the surface properties of nanoparticles.
- Polymer Science: Analyzing the surface chemistry of polymers, understanding the interactions between polymers and other materials.
- Biomaterials: Studying the surface composition of biomaterials and their interactions with biological systems.
Advantages and Limitations of AES
Advantages:
- High surface sensitivity: Provides information about the top few atomic layers.
- Elemental specificity: Can identify the presence and concentration of most elements.
- High spatial resolution: Enables analysis of small areas (down to a few micrometers).
- Quantitative analysis: Can provide quantitative information about elemental composition.
- Depth profiling: Allows the determination of elemental composition as a function of depth.
Limitations:
- Surface destructive technique: Depth profiling requires ion sputtering, which can damage the sample surface.
- Vacuum requirement: Requires ultra-high vacuum conditions, limiting the analysis of air-sensitive samples.
- Charging effects: Can be problematic for insulating samples, leading to inaccurate results.
- Limited detection of light elements: The sensitivity to light elements (like hydrogen, helium, and lithium) is generally lower.
- Matrix effects: The Auger peak intensities can be influenced by the surrounding atomic environment, making quantitative analysis challenging.
Conclusion: A Powerful Tool for Surface Analysis
Auger electron spectroscopy stands as a cornerstone technique in surface analysis, offering valuable insights into the elemental composition and structure of surfaces. By understanding the underlying principles, instrumentation, data analysis, and applications, researchers and engineers can harness the power of AES to tackle a wide range of scientific and technological challenges. While limitations exist, the advantages of its high surface sensitivity, elemental specificity, and spatial resolution solidify its indispensable role in advancing our understanding of materials and their interactions. The continued development of AES instrumentation and data analysis techniques will ensure its continued relevance and importance in future scientific discoveries.
Latest Posts
Latest Posts
-
What Are Elements Of A Culture
Mar 23, 2025
-
Why Is Interphase The Longest Phase
Mar 23, 2025
-
What Factors Influence The Rate Of Diffusion
Mar 23, 2025
-
How To Put Out A Magnesium Fire
Mar 23, 2025
-
What Is The Electron Acceptor In Fermentation
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Does Auger Electron Spectroscopy Work . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
