What Is The Electron Acceptor In Fermentation
Muz Play
Mar 23, 2025 · 6 min read
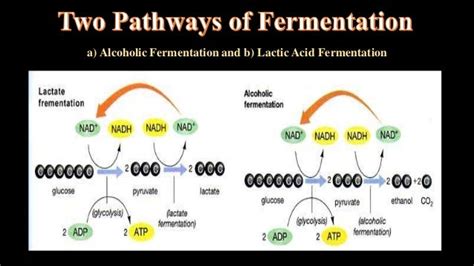
Table of Contents
What is the Electron Acceptor in Fermentation? Understanding the Process and its Variations
Fermentation, a cornerstone of metabolic processes in various organisms, often sparks confusion regarding its electron acceptor. Unlike respiration, which utilizes oxygen or other inorganic molecules as terminal electron acceptors in the electron transport chain, fermentation presents a unique picture. This article delves deep into the intricacies of fermentation, clarifying the role of electron acceptors, and exploring the diverse strategies employed by different organisms.
The Core Concept: Electron Acceptors and Redox Reactions
Before we dive into the specifics of fermentation, let's establish a fundamental understanding of electron acceptors and redox reactions. Cellular respiration and fermentation are fundamentally driven by redox reactions, which involve the transfer of electrons from one molecule (the electron donor or reductant) to another (the electron acceptor or oxidant). In respiration, the high-energy electrons derived from the breakdown of glucose are passed along an electron transport chain, ultimately reducing a strong electron acceptor like oxygen. This process releases a substantial amount of energy, captured to synthesize ATP.
Fermentation, however, operates differently. The key distinction lies in the absence of an external electron acceptor. This means that the electrons released during the initial breakdown of glucose are not passed to an external molecule. Instead, they are used to reduce an organic molecule within the cell itself. This is where the common misconception arises; there isn't a single, universal electron acceptor in fermentation. The specific organic molecule that accepts the electrons varies depending on the type of fermentation and the organism performing it.
The Absence of an External Electron Acceptor: A Defining Feature of Fermentation
The lack of an external electron acceptor is crucial in distinguishing fermentation from respiration. Respiration utilizes a strong external electron acceptor, allowing for complete oxidation of glucose and significantly higher ATP production. In contrast, fermentation's incomplete oxidation of glucose generates far less ATP. This lower energy yield is a direct consequence of the absence of an external electron acceptor and the reliance on internal organic molecules for electron acceptance.
The limited ATP production in fermentation is compensated by its speed and efficiency in anaerobic conditions where external electron acceptors are unavailable. This makes fermentation vital for organisms that thrive in oxygen-poor environments or during periods of intense activity when oxygen supply is limited.
Common Organic Electron Acceptors in Different Types of Fermentation
Several different organic molecules can function as electron acceptors in fermentation. The choice of electron acceptor often dictates the type of fermentation and the end-products generated. Let's explore some prominent examples:
1. Pyruvate in Lactic Acid Fermentation
One of the most familiar types of fermentation, lactic acid fermentation, directly uses pyruvate as the electron acceptor. In this process, NADH, the electron carrier generated during glycolysis, reduces pyruvate to lactate. This regeneration of NAD+ is essential for maintaining the continuation of glycolysis, the primary pathway for ATP production in fermentation. This is crucial as glycolysis requires NAD+ as a coenzyme. Without the reduction of pyruvate, glycolysis would halt. This process is characteristic of many bacteria (like Lactobacillus) and is also crucial in muscle cells under anaerobic conditions.
2. Acetaldehyde in Alcoholic Fermentation
Alcoholic fermentation, commonly used by yeasts and some bacteria, employs acetaldehyde as the electron acceptor. Similar to lactic acid fermentation, the goal here is to regenerate NAD+ for glycolysis. In this case, NADH reduces acetaldehyde to ethanol, effectively regenerating NAD+ and enabling the continuation of glucose breakdown. The end-products of this fermentation are ethanol and carbon dioxide, both significant in various industrial applications, like baking and brewing.
3. Other Organic Molecules in Mixed-Acid Fermentation
Mixed-acid fermentation, prevalent in certain bacteria like Escherichia coli, is more complex. It involves the production of a mixture of organic acids, such as lactate, acetate, formate, succinate, and ethanol, alongside carbon dioxide and hydrogen gas. In this case, multiple organic molecules function as electron acceptors, leading to a diverse array of end-products. The specific ratio of end-products depends on various factors, including the available nutrients and environmental conditions.
4. Understanding the Role of NADH and NAD+
The coenzymes NADH and NAD+ play a critical role in all types of fermentation. During glycolysis, NAD+ accepts electrons from glyceraldehyde-3-phosphate, converting itself to NADH. The regeneration of NAD+ is absolutely essential for glycolysis to continue. If NADH accumulates, it inhibits glycolysis. Thus, the reduction of pyruvate (or acetaldehyde, or other organic molecules) is not just about accepting electrons; it's fundamentally about recycling NAD+ to sustain ATP production via glycolysis.
Fermentation vs. Respiration: A Comparative Overview
To solidify our understanding of fermentation, it's beneficial to contrast it with respiration. The core difference, as already discussed, lies in the electron acceptor.
| Feature | Fermentation | Respiration |
|---|---|---|
| Electron Acceptor | Organic molecule (internal) | Inorganic molecule (external, e.g., O2) |
| ATP Production | Low (2 ATP per glucose molecule) | High (36-38 ATP per glucose molecule) |
| Electron Transport Chain | Absent | Present |
| Oxygen Requirement | Anaerobic (no oxygen required) | Aerobic (oxygen required) or anaerobic (using alternative electron acceptors) |
| End Products | Organic acids, alcohols, gases | Carbon dioxide and water (in aerobic respiration) |
The Importance of Fermentation in Diverse Biological Systems
Fermentation's significance extends far beyond the confines of microbiology textbooks. It plays a critical role in various aspects of life:
-
Food Production: Fermentation underpins the production of numerous foods, including yogurt, cheese, sauerkraut, kimchi, bread, and alcoholic beverages. The unique flavors and textures of these foods are a direct consequence of the metabolic activities of microorganisms during fermentation.
-
Industrial Applications: Beyond food, fermentation processes are employed in the production of various industrially significant compounds, including solvents, pharmaceuticals, and biofuels. The ability to produce these compounds efficiently and sustainably through fermentation makes it an attractive alternative to traditional chemical synthesis.
-
Human Metabolism: Lactic acid fermentation is crucial in human muscle cells during periods of intense activity when oxygen supply is limited. This process allows muscles to continue functioning even in the absence of sufficient oxygen.
-
Microbial Ecology: Many microorganisms in various environments, particularly those lacking oxygen, rely on fermentation for energy production. This process plays a critical role in nutrient cycling and decomposition within ecosystems.
Exploring the Diversity of Fermentation Pathways: Beyond the Basics
While lactic acid and alcoholic fermentations are frequently highlighted, numerous other types of fermentation exist. These variations often differ in the specific electron acceptors used and the end-products generated. Some examples include:
-
Propionic acid fermentation: Produces propionic acid, acetic acid, and carbon dioxide. Important in cheese ripening.
-
Butyric acid fermentation: Produces butyric acid, butanol, acetone, and carbon dioxide. Associated with spoilage of food.
-
Butanediol fermentation: Produces butanediol, ethanol, lactic acid, acetic acid, carbon dioxide, and hydrogen gas.
Conclusion: A Complex and Vital Metabolic Process
The question of the electron acceptor in fermentation highlights the complexity and diversity of this essential metabolic process. While there isn't a single, universal answer, understanding the role of various organic molecules as electron acceptors, the critical role of NADH and NAD+, and the overall contrast with respiration provides a comprehensive picture of fermentation. This process, far from being a simple backup mechanism to respiration, plays a vital role in various biological systems, impacting food production, industrial applications, human health, and the functioning of diverse ecosystems. Continued research continues to illuminate the fascinating intricacies of fermentation pathways and their widespread implications.
Latest Posts
Latest Posts
-
Is Water A Homogeneous Or Heterogeneous
Mar 25, 2025
-
What Is A Negatively Charged Ion Called
Mar 25, 2025
-
Changing The Ph Can Cause A Protein To
Mar 25, 2025
-
How Is Global Stratification Different From Social Stratification
Mar 25, 2025
-
Integration Of Even And Odd Functions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Acceptor In Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
