How Does Not Reaching The Equilibrium Impact Dissolution Rate
Muz Play
Mar 15, 2025 · 6 min read
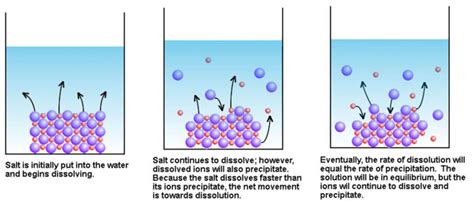
Table of Contents
How Does Not Reaching Equilibrium Impact Dissolution Rate?
Dissolution, the process by which a solid substance dissolves into a solvent to form a solution, is a fundamental concept in numerous scientific fields, including pharmaceutical sciences, environmental chemistry, and materials science. Understanding the kinetics of dissolution is crucial for optimizing various processes, from drug delivery to environmental remediation. A key aspect of dissolution is the concept of equilibrium, and deviations from this equilibrium significantly affect the dissolution rate. This article delves deep into the intricate relationship between equilibrium and dissolution rate, exploring the factors that prevent equilibrium from being reached and the consequences of this disequilibrium.
Understanding Dissolution Equilibrium
Before examining the impacts of not reaching equilibrium, it’s vital to grasp the concept of dissolution equilibrium. Dissolution is a dynamic process; it’s not a one-way street. Simultaneously with the dissolution of the solid, the dissolved molecules can precipitate back onto the solid surface. When the rate of dissolution equals the rate of precipitation, the system reaches equilibrium, also known as saturation. At this point, the concentration of the dissolved solute remains constant, although the processes of dissolution and precipitation continue at equal rates. The concentration at saturation is defined as the solubility of the substance.
Factors Affecting Equilibrium Solubility
Several factors influence the equilibrium solubility of a substance:
-
Temperature: Solubility is often temperature-dependent. For many solids dissolving in liquids, solubility increases with temperature. However, this is not always the case, and some substances exhibit inverse solubility behavior.
-
Pressure: Pressure primarily affects the solubility of gases in liquids. Increased pressure generally increases the solubility of gases. The effect on the solubility of solids is negligible under normal conditions.
-
Solvent Properties: The nature of the solvent significantly affects solubility. "Like dissolves like" is a common adage – polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. The dielectric constant and hydrogen-bonding capacity of the solvent are crucial factors.
-
pH: The pH of the solvent can significantly impact the solubility of weak acids and bases. Adjusting the pH can alter the ionization state of the solute, influencing its solubility.
-
Common Ion Effect: The presence of a common ion in the solution can reduce the solubility of a sparingly soluble salt. This is due to the Le Chatelier's principle, which states that a system at equilibrium will shift to counteract any stress applied to it.
The Impact of Not Reaching Equilibrium on Dissolution Rate
When equilibrium is not reached, the dissolution rate is directly affected. The dissolution rate, often expressed as the change in concentration per unit time, continues to increase until equilibrium is established (or until the solid is completely dissolved). This is because the driving force for dissolution, the concentration gradient, remains high. The concentration gradient is the difference between the concentration of the solute at the solid-liquid interface and the concentration of the solute in the bulk solution. A higher concentration gradient leads to a faster dissolution rate.
Factors Preventing Equilibrium
Several factors can prevent a system from reaching dissolution equilibrium:
-
Limited Contact Time: If the contact time between the solid and the solvent is insufficient, the system may not have enough time to reach equilibrium. This is often the case in rapidly flowing systems or in processes with short durations.
-
Unsuitable Mixing: Inadequate mixing can lead to the formation of a boundary layer around the solid, limiting the diffusion of dissolved molecules away from the surface. This boundary layer acts as a barrier, hindering the dissolution process and preventing the system from reaching equilibrium.
-
Slow Dissolution Kinetics: Some substances have inherently slow dissolution rates, meaning that even with ample contact time and good mixing, equilibrium may not be reached within a reasonable timeframe. This can be due to factors such as the crystal structure of the solid, the presence of impurities, or the formation of insoluble surface layers.
-
Supersaturation: If the concentration of the solute in the solution exceeds its equilibrium solubility, the solution becomes supersaturated. In this state, precipitation will occur to reduce the concentration back to the equilibrium solubility. However, supersaturation can persist for some time, especially in the absence of nucleation sites for crystal growth.
-
Chemical Reactions: If the dissolved solute undergoes a chemical reaction in the solution, it might prevent the system from reaching equilibrium. For instance, if the solute reacts with the solvent or other components of the solution, the concentration of the undissolved solute will decrease more rapidly than it would without the reaction.
Consequences of Disequilibrium
The failure to reach equilibrium has several significant consequences:
-
Inaccurate Solubility Measurements: If the dissolution process is not allowed to reach equilibrium, any measurements of solubility will be underestimated. This can have serious implications in various applications where accurate solubility data is crucial.
-
Incomplete Drug Dissolution: In pharmaceutical applications, incomplete drug dissolution can lead to suboptimal therapeutic efficacy. If a drug does not dissolve sufficiently in the gastrointestinal tract, it cannot be absorbed and exert its intended effect.
-
Environmental Concerns: In environmental remediation, incomplete dissolution can result in persistent contamination. If a pollutant does not fully dissolve, it can continue to contaminate the environment for an extended period.
-
Process Optimization Challenges: Understanding the factors that prevent the attainment of equilibrium is critical for optimizing processes. If equilibrium is not attained, processes might be less efficient, leading to increased costs and resource consumption.
Strategies to Enhance Dissolution Rate and Achieve Equilibrium
Several strategies can be employed to enhance the dissolution rate and increase the likelihood of reaching equilibrium:
-
Particle Size Reduction: Decreasing the particle size of the solid increases the surface area available for dissolution, significantly enhancing the rate. Techniques like milling and micronization are commonly used.
-
Polymorphism Control: Different crystalline forms (polymorphs) of the same substance can exhibit different solubilities and dissolution rates. Selecting the appropriate polymorph can enhance dissolution.
-
Solid Dispersions: Incorporating the drug into a solid dispersion can improve its wettability and dissolution rate.
-
Surfactants: Adding surfactants to the solvent can reduce the interfacial tension between the solid and the liquid, improving wettability and enhancing dissolution.
-
Cosolvents: Using cosolvents, which are solvents that enhance the solubility of a solute in a primary solvent, can increase the dissolution rate.
-
Improved Mixing and Agitation: Efficient mixing and agitation ensure that the concentration gradient is maintained, driving dissolution and promoting the attainment of equilibrium.
-
Controlled Release Formulations: For pharmaceutical applications, controlled release formulations are designed to release the drug at a controlled rate, ensuring that therapeutic levels are maintained without causing sudden fluctuations in concentration.
Conclusion
The attainment of dissolution equilibrium is crucial for accurate solubility measurements and the successful application of dissolution processes in various fields. Factors such as limited contact time, poor mixing, slow kinetics, supersaturation, and chemical reactions can hinder the achievement of equilibrium. Understanding these factors and employing appropriate strategies, such as particle size reduction, polymorphism control, and the use of surfactants, can enhance the dissolution rate and help in achieving equilibrium. The consequences of not reaching equilibrium can be significant, ranging from inaccurate data to suboptimal process performance and environmental concerns. Therefore, a thorough understanding of the dynamics of dissolution and the factors that influence it is paramount for efficient and successful implementation in various scientific and technological applications. Further research into the intricacies of dissolution kinetics continues to provide valuable insights and improve strategies for optimizing dissolution processes in diverse fields.
Latest Posts
Latest Posts
-
Which One Neutral Charge Proton Or Neutron
Mar 15, 2025
-
A Solids Volume And Shape Is Defintie
Mar 15, 2025
-
How Many Protons Does Iodine Have
Mar 15, 2025
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Does Not Reaching The Equilibrium Impact Dissolution Rate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
