How Many Protons Does Iodine Have
Muz Play
Mar 15, 2025 · 5 min read
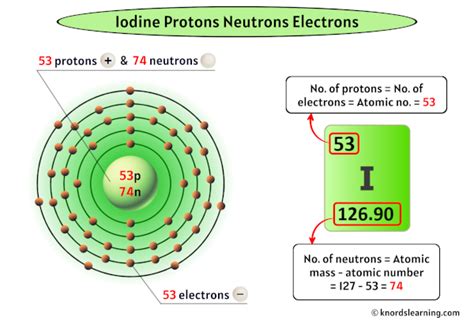
Table of Contents
How Many Protons Does Iodine Have? A Deep Dive into Atomic Structure
Iodine, a fascinating element crucial for human health and various industrial applications, holds a unique place in the periodic table. Understanding its fundamental properties, particularly its atomic structure, is key to appreciating its role in the world around us. This in-depth article will explore the question: How many protons does iodine have? We'll delve beyond a simple numerical answer to explore the broader implications of this number, its relation to iodine's properties, and its significance in chemistry and beyond.
Unveiling the Atomic Heart: Protons, Neutrons, and Electrons
Before we pinpoint the number of protons in iodine, let's establish a foundational understanding of atomic structure. Atoms, the fundamental building blocks of matter, are composed of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutrally charged particles also residing in the nucleus. Their number can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
Understanding the interplay of these particles is crucial for comprehending an element's chemical behavior and physical properties.
Iodine's Atomic Identity: The Defining Number of Protons
The answer to our central question is straightforward: Iodine (I) has 53 protons. This number, 53, is iodine's atomic number, a unique identifier that distinguishes it from all other elements. This fundamental characteristic dictates iodine's placement on the periodic table and fundamentally shapes its chemical and physical properties. No other element possesses 53 protons; this number is exclusive to iodine.
The Significance of Atomic Number
The atomic number is not merely a label; it's a deeply significant quantity:
- Elemental Identity: The number of protons unequivocally defines the element. A change in the number of protons transforms the element entirely.
- Chemical Behavior: The number of protons dictates the number of electrons in a neutral atom, directly influencing its electron configuration and, consequently, its chemical reactivity. Iodine's 53 electrons are arranged in specific shells, determining its bonding capabilities and its tendency to form compounds.
- Periodic Table Organization: The periodic table is organized by increasing atomic number, reflecting the fundamental nature of this quantity in classifying elements. Iodine's position reflects its properties and relationships with its neighboring elements.
Isotopes of Iodine: Variations on a Theme
While the number of protons remains constant (53) for iodine, the number of neutrons can vary. These variations create different isotopes of iodine. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This affects the atom's mass but not its chemical properties significantly.
Several iodine isotopes exist, including:
- Iodine-127 (¹²⁷I): This is the most stable and abundant isotope of iodine, comprising almost 100% of naturally occurring iodine. Its mass number (protons + neutrons) is 127.
- Iodine-131 (¹³¹I): A radioactive isotope used in medical applications, such as treating thyroid disorders. Its mass number is 131. This isotope's radioactivity stems from its unstable neutron-to-proton ratio.
The existence of isotopes highlights that while the number of protons defines the element, variations in the number of neutrons can lead to different properties, particularly regarding nuclear stability and radioactivity.
Iodine's Properties: A Consequence of its Atomic Structure
Iodine's properties, both physical and chemical, are directly linked to its atomic structure and, specifically, its 53 protons.
Physical Properties:
- Appearance: Solid at room temperature, exhibiting a dark gray to bluish-black metallic luster.
- State: Sublimes readily, meaning it transitions directly from a solid to a gas without an intermediate liquid phase. This property is crucial in certain applications.
- Solubility: Slightly soluble in water but readily soluble in nonpolar solvents. This solubility characteristic influences its interactions with various substances.
Chemical Properties:
- Halogen Family: Iodine is a halogen, a group of highly reactive nonmetals. Its reactivity is a consequence of its electron configuration; it readily gains one electron to achieve a stable electron configuration.
- Oxidation States: Iodine exhibits various oxidation states, reflecting its ability to gain or lose electrons in chemical reactions. This versatility contributes to its diverse chemical behavior.
- Compound Formation: Iodine forms various compounds, particularly with metals and other nonmetals, demonstrating its capacity to participate in a wide range of chemical reactions.
The 53 protons in iodine's nucleus directly influence its electron configuration, driving its distinctive physical and chemical properties.
Iodine's Role in Biology and Medicine: A Vital Element
Iodine's importance extends beyond the realm of chemistry. It plays a vital role in human biology and medicine:
- Thyroid Hormone Synthesis: Iodine is essential for the synthesis of thyroid hormones, thyroxine (T4) and triiodothyronine (T3). These hormones regulate metabolism, growth, and development. Iodine deficiency can lead to goiter, hypothyroidism, and other health problems. The body's requirement for iodine highlights the element's critical biological function.
- Medical Imaging and Treatment: Radioactive iodine-131 is used in medical imaging techniques and in the treatment of thyroid cancer. This application exploits the thyroid gland's affinity for iodine.
- Antiseptic Properties: Iodine exhibits antiseptic properties, used in disinfectants and wound treatments. This stems from its ability to kill bacteria and other microorganisms.
Applications Beyond Biology: Diverse Industrial Uses
Iodine's unique properties also find applications in diverse industrial settings:
- Photography: Historically used in photographic processes due to its light-sensitive properties.
- Catalysis: Used as a catalyst in various chemical reactions.
- Dyes and Pigments: Used in the production of various dyes and pigments.
- Livestock Feed: Added to livestock feed to supplement iodine intake.
Conclusion: The Significance of 53 Protons
The simple answer – iodine has 53 protons – unveils a rich tapestry of scientific understanding. This number defines iodine as a unique element, dictates its chemical and physical properties, determines its biological role, and underlies its diverse industrial applications. Understanding the fundamental nature of atomic structure, particularly the significance of the atomic number, provides a deeper appreciation of iodine's crucial role in our world. The number 53 is not just a numerical value; it is the key to unlocking the properties and importance of this fascinating element. It represents the foundation of iodine's identity and its diverse impact on our lives. From the intricacies of thyroid hormone synthesis to its application in medical treatments and industrial processes, the 53 protons in iodine's nucleus are a testament to the power of fundamental science and its influence on our world.
Latest Posts
Latest Posts
-
Columns Of The Periodic Table Are Called
Mar 15, 2025
-
Is Boiling Point Intensive Or Extensive
Mar 15, 2025
-
A Triple Bond Is Generally Composed Of
Mar 15, 2025
-
Actin Filaments Are Anchored To Structures Called
Mar 15, 2025
-
How To Identify A Weak Acid
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Does Iodine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
