How Does Polarity Affect Boiling Point
Muz Play
Mar 18, 2025 · 5 min read
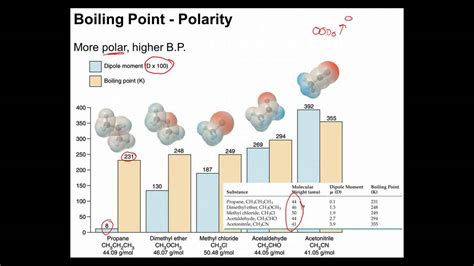
Table of Contents
How Does Polarity Affect Boiling Point? A Deep Dive into Intermolecular Forces
Understanding the relationship between polarity and boiling point is crucial in chemistry. It's a fundamental concept that explains why some substances boil at incredibly low temperatures while others require significantly higher temperatures. This comprehensive guide delves into the intricate details of intermolecular forces, explaining how polarity, a measure of a molecule's uneven charge distribution, dramatically influences a substance's boiling point.
The Essence of Boiling: Overcoming Intermolecular Forces
Before we explore the role of polarity, let's establish a foundational understanding of boiling. Boiling is the phase transition where a liquid transforms into a gas. This transition doesn't occur spontaneously; it necessitates overcoming the intermolecular forces holding the liquid molecules together. These forces are the attractive interactions between molecules, and their strength directly dictates the boiling point. The stronger these forces, the more energy (and thus higher temperature) is required to break them and allow the molecules to escape into the gaseous phase.
Types of Intermolecular Forces: A Hierarchy of Attraction
Several types of intermolecular forces exist, each with varying strengths:
-
London Dispersion Forces (LDFs): These are the weakest intermolecular forces and are present in all molecules, regardless of polarity. They arise from temporary, instantaneous dipoles created by the fluctuating electron distribution within a molecule. Larger, more complex molecules generally exhibit stronger LDFs due to their increased number of electrons and larger surface area.
-
Dipole-Dipole Interactions: These forces occur between polar molecules – molecules with a permanent dipole moment due to an uneven distribution of electron density. The positive end of one polar molecule is attracted to the negative end of another. Dipole-dipole interactions are stronger than LDFs.
-
Hydrogen Bonding: This is a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine). The highly electronegative atom attracts the electron density away from the hydrogen, creating a strong partial positive charge on the hydrogen. This partially positive hydrogen is then strongly attracted to the lone pairs of electrons on another electronegative atom in a nearby molecule. Hydrogen bonds are significantly stronger than typical dipole-dipole interactions and are responsible for many of the unique properties of water.
Polarity: The Key Player
Polarity, as mentioned earlier, refers to the uneven distribution of electron density within a molecule. This uneven distribution results from differences in the electronegativity of the atoms within the molecule. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. A large difference in electronegativity between atoms in a molecule leads to a significant dipole moment, resulting in a highly polar molecule.
How Polarity Impacts Intermolecular Forces
The impact of polarity on boiling point is directly linked to its effect on intermolecular forces:
-
Nonpolar Molecules: Nonpolar molecules only experience London Dispersion Forces (LDFs). The strength of these forces increases with the size and molecular weight of the molecule. Therefore, nonpolar molecules generally have low boiling points, especially those with smaller molecular weights.
-
Polar Molecules: Polar molecules experience both LDFs and dipole-dipole interactions. The addition of dipole-dipole interactions significantly increases the strength of the intermolecular forces compared to nonpolar molecules of similar size. This translates to a higher boiling point for polar molecules.
-
Hydrogen-Bonded Molecules: Molecules capable of hydrogen bonding exhibit the strongest intermolecular forces. Hydrogen bonds dramatically increase the boiling point compared to molecules of similar size and polarity that do not participate in hydrogen bonding. Water (H₂O), for example, has an exceptionally high boiling point for its molecular weight due to its extensive hydrogen bonding network.
Examples Illustrating the Polarity-Boiling Point Relationship
Let's consider some examples to solidify our understanding:
1. Methane (CH₄) vs. Water (H₂O):
Methane is a nonpolar molecule, relying solely on LDFs for intermolecular attraction. Its boiling point is -161.5 °C. Water, on the other hand, is a polar molecule with extensive hydrogen bonding. Its boiling point is 100 °C, a stark contrast highlighting the significant impact of hydrogen bonding.
2. Ethanol (CH₃CH₂OH) vs. Dimethyl Ether (CH₃OCH₃):
Both ethanol and dimethyl ether have the same molecular formula (C₂H₆O) but differ in their structures and polarity. Ethanol is polar due to the presence of the hydroxyl (-OH) group, which allows for hydrogen bonding. Dimethyl ether, however, is less polar, lacking the ability to form hydrogen bonds. Ethanol's boiling point (78.4 °C) is significantly higher than dimethyl ether's (-23.6 °C), demonstrating the effect of hydrogen bonding.
3. Butane (C₄H₁₀) vs. 1-Chlorobutane (C₄H₉Cl):
Butane is a nonpolar alkane. 1-chlorobutane is polar due to the presence of the electronegative chlorine atom. While both molecules have comparable molecular weights, the dipole-dipole interactions in 1-chlorobutane lead to a substantially higher boiling point than butane.
4. Analyzing Isomers:
Isomers are molecules with the same molecular formula but different structural arrangements. Isomers can exhibit different polarities, which directly affect their boiling points. For instance, consider n-butane and isobutane. N-butane, with its linear structure, has a slightly higher boiling point than isobutane due to its stronger London Dispersion Forces resulting from a more elongated shape.
Factors Beyond Polarity: A Holistic View
While polarity is a primary determinant of boiling point, it's not the only factor. Other aspects also contribute:
-
Molecular Weight: Larger molecules generally have stronger LDFs, leading to higher boiling points, even if they are nonpolar.
-
Molecular Shape: The shape of a molecule influences the extent of surface contact between molecules, impacting the strength of LDFs. More compact molecules often have lower boiling points than their linear counterparts.
-
Branching: Branching reduces the surface area available for intermolecular interactions, leading to weaker LDFs and lower boiling points.
Conclusion: A Comprehensive Understanding
The boiling point of a substance is a direct consequence of the strength of its intermolecular forces. Polarity plays a crucial role in determining the strength of these forces. Polar molecules exhibit stronger intermolecular forces (dipole-dipole and potentially hydrogen bonding) than nonpolar molecules, which rely solely on LDFs. This difference directly translates into higher boiling points for polar substances. While polarity is a key factor, molecular weight, shape, and branching also influence the boiling point. Understanding the interplay of these factors provides a comprehensive understanding of the physical properties of substances and their behavior at different temperatures.
Latest Posts
Latest Posts
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Does Polarity Affect Boiling Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
