How Does Temperature Affect Rate Of Diffusion
Muz Play
Mar 25, 2025 · 6 min read
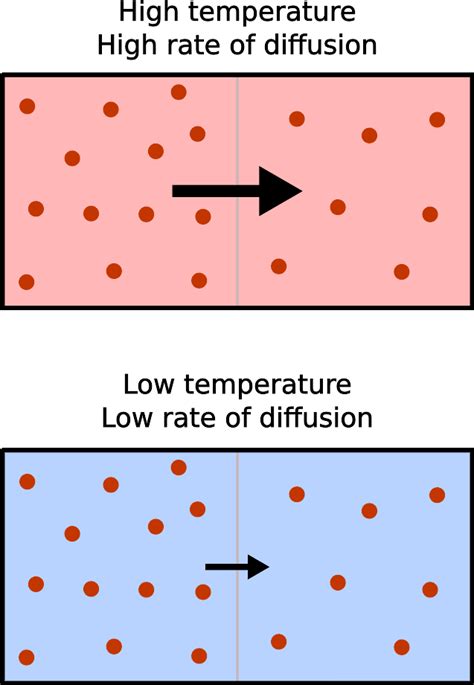
Table of Contents
How Does Temperature Affect the Rate of Diffusion?
Diffusion, the net movement of particles from a region of higher concentration to a region of lower concentration, is a fundamental process in many natural phenomena and technological applications. Understanding the factors that influence the rate of diffusion is crucial in various fields, from biology and chemistry to materials science and engineering. One of the most significant factors affecting diffusion rate is temperature. This article will delve into the intricate relationship between temperature and diffusion rate, exploring the underlying mechanisms and practical implications.
The Kinetic Theory of Gases and Diffusion
To understand the impact of temperature on diffusion, we must first consider the kinetic theory of gases. This theory posits that matter is made up of tiny particles (atoms or molecules) in constant, random motion. The kinetic energy of these particles is directly proportional to the absolute temperature. This means that as temperature increases, the particles move faster.
Increased Kinetic Energy: The Driving Force Behind Faster Diffusion
Higher temperatures translate to higher average kinetic energies of the diffusing particles. This increased kinetic energy means the particles possess greater velocity and undergo more frequent collisions. These more energetic and frequent collisions lead to a more rapid spread of the particles, thereby increasing the rate of diffusion.
Visualizing the Effect: A Simple Analogy
Imagine dropping a drop of food coloring into a glass of water. At room temperature, the color gradually spreads, taking some time to fully disperse. Now, imagine heating the water. The color will diffuse much faster because the warmer water molecules move more rapidly, distributing the food coloring molecules more efficiently.
Temperature's Influence on Diffusion in Different States of Matter
Temperature's effect on diffusion isn't limited to gases. While the effect is most pronounced in gases due to their loosely bound particles, it also plays a significant role in liquids and solids, albeit with some nuanced differences.
Diffusion in Gases: A Significant Temperature Dependence
In gases, the particles are far apart and interact weakly, making temperature the dominant factor controlling diffusion. A rise in temperature directly translates into a substantial increase in the diffusion rate, often following an exponential relationship. This is because the increase in kinetic energy significantly overcomes the weak intermolecular forces, allowing for rapid particle movement.
Diffusion in Liquids: A More Complex Relationship
In liquids, the particles are closer together and experience stronger intermolecular forces. While temperature still increases kinetic energy and thus particle velocity, the stronger intermolecular forces partially restrict the movement of particles. The increase in diffusion rate with temperature is still observed in liquids, but it's typically less dramatic than in gases. The viscosity of the liquid also plays a role, with less viscous liquids showing a more pronounced temperature dependence on diffusion.
Diffusion in Solids: Temperature's Subtle Yet Crucial Role
In solids, the particles are tightly packed and held in place by strong intermolecular forces. Diffusion in solids is a significantly slower process compared to gases and liquids. However, temperature still influences the diffusion rate. Increasing the temperature provides the particles with sufficient energy to overcome the strong intermolecular forces and "jump" into adjacent sites within the solid lattice structure. This phenomenon is known as vacancy diffusion or interstitial diffusion, depending on the mechanism. While the increase in diffusion rate with temperature is less pronounced in solids than in gases and liquids, it's crucial for processes like heat treatment and material processing.
Mathematical Representation: Fick's First Law and Temperature Dependence
The relationship between temperature and diffusion rate is often described mathematically using Fick's First Law of Diffusion. This law states that the diffusion flux (the amount of substance diffusing per unit area per unit time) is proportional to the concentration gradient. The proportionality constant is the diffusion coefficient (D), which is heavily dependent on temperature.
The diffusion coefficient often follows the Arrhenius equation, which expresses the temperature dependence exponentially:
D = D₀ * exp(-Ea / RT)
Where:
- D is the diffusion coefficient
- D₀ is a pre-exponential factor (temperature-independent)
- Ea is the activation energy for diffusion (the energy barrier particles must overcome to diffuse)
- R is the ideal gas constant
- T is the absolute temperature
This equation highlights the exponential relationship between the diffusion coefficient (and thus the diffusion rate) and temperature. A small increase in temperature can lead to a significant increase in the diffusion coefficient, especially at lower temperatures.
Practical Implications of Temperature's Effect on Diffusion
The temperature dependence of diffusion has numerous practical implications across various fields:
Material Science and Engineering: Heat Treatment and Diffusion Bonding
Heat treatment processes in metallurgy rely heavily on the temperature dependence of diffusion. Annealing, hardening, and tempering all involve controlled heating and cooling cycles to manipulate the diffusion of atoms within the material, resulting in desired properties like strength, hardness, and ductility. Similarly, diffusion bonding involves using high temperatures to allow atomic diffusion at the interface between two materials, creating a strong bond without melting the materials.
Biology and Medicine: Enzyme Activity and Drug Delivery
Temperature plays a critical role in biological processes involving diffusion. Enzyme activity, a crucial aspect of metabolism, is highly temperature-dependent, with optimal activity occurring within a specific temperature range. Outside this range, enzyme activity diminishes, impacting biological processes. In drug delivery systems, temperature influences the rate at which drugs diffuse through tissues and membranes, impacting drug efficacy and absorption.
Environmental Science: Pollutant Dispersion and Atmospheric Mixing
Diffusion plays a significant role in the dispersion of pollutants in the environment. Temperature gradients in the atmosphere affect the mixing rate of pollutants, influencing their concentration levels and impacting air quality. Higher temperatures often lead to faster mixing, but complex atmospheric dynamics also influence pollutant dispersion.
Food Science: Preservation and Flavor Development
Temperature influences the diffusion of aroma compounds and other molecules in food, impacting flavor and texture. Preservation techniques like refrigeration and freezing rely on the slower diffusion rates at lower temperatures to slow down microbial growth and enzymatic activity.
Conclusion: Temperature – A Master Regulator of Diffusion
Temperature exerts a profound influence on the rate of diffusion across all states of matter. The relationship, often described by the Arrhenius equation, is exponential, indicating that even small changes in temperature can significantly affect diffusion rates. This fundamental principle has widespread implications across diverse fields, from materials science and engineering to biology and environmental science. A thorough understanding of this relationship is essential for optimizing processes, designing new materials, and predicting the behavior of systems involving diffusion. Further research continues to refine our understanding of this complex phenomenon, leading to innovative applications and advancements in various scientific disciplines.
Latest Posts
Latest Posts
-
What Controls What Enters And Leaves A Cell
Mar 28, 2025
-
Time Rate Of Change Of Momentum
Mar 28, 2025
-
Definition Of Marketing By Philip Kotler
Mar 28, 2025
-
Formulas For Photosynthesis And Cellular Respiration
Mar 28, 2025
-
The Blank Atom In R 12 Is Believed To Brea Off
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about How Does Temperature Affect Rate Of Diffusion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
