How Is A Double Bond Treated In Vsepr
Muz Play
Mar 23, 2025 · 6 min read
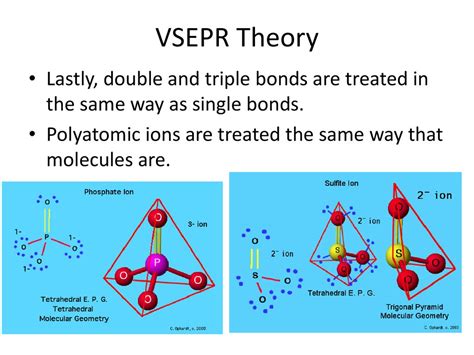
Table of Contents
How is a Double Bond Treated in VSEPR Theory?
Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting the three-dimensional shapes of molecules. It's based on the simple principle that electron pairs, whether bonding or non-bonding (lone pairs), repel each other and will arrange themselves to be as far apart as possible to minimize this repulsion. Understanding how double bonds are treated within this framework is crucial for accurately predicting molecular geometry. This article will delve into the nuances of double bond treatment in VSEPR, exploring various examples and clarifying common misconceptions.
The VSEPR Core Principle: Electron Pair Repulsion
At the heart of VSEPR lies the concept of electron pair repulsion. Both bonding and non-bonding electron pairs influence the molecular shape. However, the type of electron pair – single, double, or triple – affects the strength of repulsion, and consequently, the final geometry.
Single, Double, and Triple Bonds: A Comparison
While VSEPR treats all electron pairs as occupying space, it's important to understand the differences in electron density:
- Single Bond: One sigma (σ) bond, relatively localized electron density.
- Double Bond: One sigma (σ) bond and one pi (π) bond. The π bond has higher electron density concentrated above and below the internuclear axis.
- Triple Bond: One sigma (σ) bond and two pi (π) bonds. Even higher electron density concentrated above and below the internuclear axis than in a double bond.
Although the π bonds contribute to stronger repulsion compared to a σ bond, VSEPR treats double and triple bonds as single effective electron domains. This is a crucial point to remember. The extra electron density in the multiple bonds leads to slightly shorter bond lengths and stronger repulsive forces, but it doesn't change the number of regions of electron density around the central atom that determine the basic geometry.
Applying VSEPR to Molecules with Double Bonds
The process of predicting the shape of a molecule using VSEPR, even with double bonds, remains consistent:
-
Draw the Lewis Structure: This step is fundamental. It allows you to determine the number of electron pairs (bonding and non-bonding) around the central atom.
-
Count Electron Domains: Each single, double, or triple bond counts as one electron domain. Lone pairs also count as one electron domain each.
-
Determine the Electron Domain Geometry: This refers to the arrangement of electron domains around the central atom. Based on the number of electron domains, we have the following geometries:
- 2 Electron Domains: Linear
- 3 Electron Domains: Trigonal Planar
- 4 Electron Domains: Tetrahedral
- 5 Electron Domains: Trigonal Bipyramidal
- 6 Electron Domains: Octahedral
-
Determine the Molecular Geometry: This refers to the arrangement of atoms around the central atom, taking into account the presence of lone pairs. Lone pairs exert stronger repulsive forces than bonding pairs, often causing deviations from ideal geometries.
Examples: Illustrating Double Bond Treatment in VSEPR
Let's examine some examples to solidify our understanding.
Example 1: Ethylene (C₂H₄)
Ethylene has a carbon-carbon double bond. Each carbon atom has three electron domains (one double bond and two single bonds). The electron domain geometry around each carbon is trigonal planar, resulting in a planar molecular geometry for the entire molecule. The H-C-H bond angle is approximately 120°.
Example 2: Carbon Dioxide (CO₂)
Carbon dioxide has two double bonds between the carbon atom and the oxygen atoms. The carbon atom has two electron domains. The electron domain geometry and the molecular geometry are both linear, with a 180° bond angle.
Example 3: Formaldehyde (H₂CO)
Formaldehyde contains a carbon-oxygen double bond and two carbon-hydrogen single bonds. The central carbon atom has three electron domains (one double bond and two single bonds). The electron domain geometry is trigonal planar, and the molecular geometry is also trigonal planar. The H-C-H bond angle is approximately 120°.
Example 4: Ozone (O₃)
Ozone presents a slightly more complex case. The central oxygen atom has three electron domains: one double bond, one single bond, and one lone pair. The electron domain geometry is trigonal planar, but the molecular geometry is bent due to the presence of the lone pair, which exerts stronger repulsion.
Example 5: Sulfuric Acid (H₂SO₄)
Sulfuric acid (H₂SO₄) provides a more complex illustration. The central sulfur atom is surrounded by four electron domains: two double bonds to oxygen and two single bonds to hydroxyl (-OH) groups. Although there are double bonds, they are each treated as a single electron domain. This results in a tetrahedral electron domain geometry and a tetrahedral molecular geometry.
Addressing Common Misconceptions
Several misunderstandings often arise when dealing with double bonds in VSEPR:
-
Double bonds don't count as two electron domains: Remember, while double bonds have a higher electron density, they occupy a single region of space around the central atom, thus contributing only one electron domain.
-
Pi bonds directly influence the molecular shape: Pi bonds don't independently dictate the molecular shape; their increased electron density is implicitly accounted for by the single electron domain designation. The overall electron domain geometry is what primarily determines the shape.
-
VSEPR is infallible: While VSEPR is a powerful predictive tool, it's not perfect. It is a simplified model that can sometimes fail to accurately predict the precise bond angles and molecular shapes of complex molecules. Other factors, such as steric hindrance and resonance structures, can influence the final geometry.
Beyond the Basics: Advanced Considerations
For a more in-depth understanding, consider these advanced topics:
-
Resonance Structures: When resonance structures exist, VSEPR predictions often still hold, though the actual bond lengths and angles might be slightly different than what the model predicts for any single contributing structure. Average bond order and electron delocalization needs to be considered.
-
Steric Effects: Bulky substituents can influence bond angles and slightly distort molecular geometries from those predicted by VSEPR.
-
Hypervalent Molecules: Molecules with central atoms exceeding the octet rule can exhibit more complex geometries. Extended VSEPR models are sometimes necessary to accommodate these situations.
Conclusion
VSEPR theory provides a simplified, yet effective, method for predicting molecular geometries. The key to accurately applying VSEPR to molecules containing double bonds is to remember that each double bond is treated as one electron domain. By systematically following the steps outlined in this article, one can confidently predict the shapes of a vast array of molecules, even those containing multiple bonds. While understanding the nuances of electron density and potential exceptions is important for more complex cases, grasping the fundamental principle of treating double bonds as single electron domains forms the solid foundation for successful VSEPR application. Remember that VSEPR is a powerful tool, but it’s crucial to utilize it alongside other theoretical models for a thorough understanding of molecular structure and behavior.
Latest Posts
Latest Posts
-
Position Of The Sun Moon And Earth
Mar 24, 2025
-
What Is The Difference Between Supportive And Defensive Communication
Mar 24, 2025
-
Is Turnover Number Affected By Substrate Concentration
Mar 24, 2025
-
One Mole Of Any Element Has The Same
Mar 24, 2025
-
Which Of The Following Is A Simple Definition Of Reduction
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Is A Double Bond Treated In Vsepr . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
