How Many Bonding Domains Does A Lone Pair Account For
Muz Play
Mar 16, 2025 · 6 min read
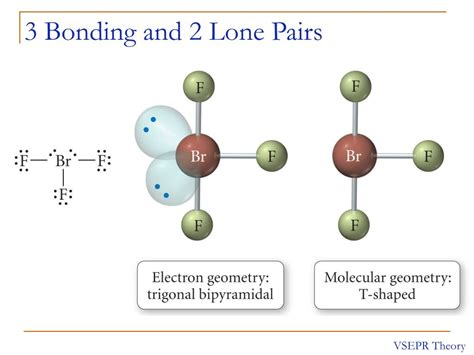
Table of Contents
How Many Bonding Domains Does a Lone Pair Account For? Understanding VSEPR Theory
The question of how many bonding domains a lone pair accounts for is a fundamental concept in understanding molecular geometry. It's crucial for predicting the shape of molecules and their resulting properties. While the answer might seem straightforward at first glance, a deeper dive reveals subtleties and nuances that are critical for accurate predictions. This article will delve into the complexities of this question, exploring VSEPR theory, its limitations, and how lone pairs influence molecular geometry.
Understanding VSEPR Theory: The Foundation of Molecular Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a simple yet powerful model for predicting the three-dimensional arrangement of atoms in a molecule. The core principle of VSEPR theory is that electron pairs, both bonding and non-bonding (lone pairs), repel each other and will arrange themselves to minimize this repulsion. This arrangement dictates the molecule's overall geometry.
Bonding Domains vs. Lone Pairs: A Key Distinction
A bonding domain represents a region of space where electrons are shared between two atoms, forming a covalent bond. A lone pair, also known as a non-bonding pair, represents a pair of electrons that are associated with only one atom. These lone pairs are not involved in bonding with other atoms. While both bonding domains and lone pairs occupy space around the central atom, they differ significantly in their influence on molecular geometry.
How Lone Pairs Affect Molecular Geometry
The key takeaway is that lone pairs occupy more space than bonding domains. This is because lone pairs are not constrained by being shared between two nuclei. They are held closer to the central atom, exerting a stronger repulsive force on bonding domains. This increased repulsion causes bond angles to be compressed compared to the ideal geometry predicted if only bonding domains were present.
The Role of Lone Pairs: Steric Number and Geometry
The steric number is the sum of the number of bonding domains and lone pairs around the central atom. This number is crucial in determining the electron-pair geometry and the molecular geometry. Let's clarify:
- Electron-pair geometry describes the arrangement of all electron pairs (bonding and lone pairs) around the central atom. This geometry is determined solely by the steric number.
- Molecular geometry describes the arrangement of only the atoms in the molecule. This is where the influence of lone pairs becomes crucial as they affect the positions of the atoms.
For example:
- Steric number 2: Two bonding domains lead to a linear electron-pair geometry and a linear molecular geometry (like BeCl₂). Two lone pairs (like in XeF₂) lead to a linear electron-pair geometry, but a linear molecular geometry (only considering the positions of the atoms).
- Steric number 3: Three bonding domains lead to a trigonal planar electron-pair geometry and a trigonal planar molecular geometry (like BF₃). Two bonding domains and one lone pair (like in SO₂) lead to a trigonal planar electron-pair geometry, but a bent molecular geometry.
- Steric number 4: Four bonding domains lead to a tetrahedral electron-pair geometry and a tetrahedral molecular geometry (like CH₄). Three bonding domains and one lone pair (like in NH₃) lead to a tetrahedral electron-pair geometry but a trigonal pyramidal molecular geometry. Two bonding domains and two lone pairs (like in H₂O) lead to a tetrahedral electron-pair geometry but a bent molecular geometry.
Crucially, a lone pair always contributes to the steric number, but it doesn't directly contribute to the molecular geometry in the same way a bonding domain does. It influences the molecular geometry by pushing the bonding domains closer together. It therefore influences the shape, but its position is not directly included in naming the shape.
Lone Pairs: Repulsive Force and Bond Angle Distortion
The stronger repulsive forces exerted by lone pairs lead to distortions in bond angles. The order of repulsive forces is generally: lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair. This means that the presence of lone pairs will always compress the bond angles between the bonding pairs.
Let's consider water (H₂O) as an example. The steric number is 4 (two bonding pairs and two lone pairs). The electron-pair geometry is tetrahedral. However, the lone pairs exert a stronger repulsive force than the bonding pairs, causing the H-O-H bond angle to be compressed from the ideal tetrahedral angle of 109.5° to approximately 104.5°.
Beyond Simple VSEPR: Limitations and Refinements
While VSEPR theory is remarkably successful in predicting molecular geometries, it has limitations. It doesn't account for:
- The size of atoms: Larger atoms can accommodate more electron pairs without significant distortion.
- Multiple bonds: Double and triple bonds occupy more space than single bonds, influencing bond angles.
- Hybridization: The concept of orbital hybridization provides a more detailed explanation of bonding and electron arrangement.
- Advanced electronic effects: Factors like electronegativity differences and hyperconjugation can further influence molecular geometry.
More sophisticated computational methods, such as Density Functional Theory (DFT) and ab initio calculations, are used to accurately predict molecular geometries in complex systems where VSEPR provides only a rough approximation.
Applying the Understanding: Examples and Case Studies
Let's illustrate the concept with a few examples:
1. Ammonia (NH₃):
- Central atom: Nitrogen (N)
- Bonding domains: 3 (N-H bonds)
- Lone pairs: 1
- Steric number: 4
- Electron-pair geometry: Tetrahedral
- Molecular geometry: Trigonal pyramidal (the lone pair pushes the three N-H bonds downwards)
2. Carbon Dioxide (CO₂):
- Central atom: Carbon (C)
- Bonding domains: 2 (C=O double bonds)
- Lone pairs: 0
- Steric number: 2
- Electron-pair geometry: Linear
- Molecular geometry: Linear
3. Sulfur Dioxide (SO₂):
- Central atom: Sulfur (S)
- Bonding domains: 2 (S=O double bonds)
- Lone pairs: 1
- Steric number: 3
- Electron-pair geometry: Trigonal planar
- Molecular geometry: Bent (the lone pair pushes the two S=O bonds closer together)
4. Xenon Tetrafluoride (XeF₄):
- Central atom: Xenon (Xe)
- Bonding domains: 4 (Xe-F bonds)
- Lone pairs: 2
- Steric number: 6
- Electron-pair geometry: Octahedral
- Molecular geometry: Square planar (the two lone pairs occupy opposite positions)
These examples highlight the importance of considering both bonding domains and lone pairs when predicting molecular geometry. The lone pairs, despite not directly forming bonds, play a significant role in determining the three-dimensional arrangement of atoms.
Conclusion: Lone Pairs and the Nuances of Molecular Geometry Prediction
In conclusion, while a lone pair does not directly contribute to the number of bonds in a molecule, it significantly impacts the molecular geometry. It contributes to the steric number, influencing the overall electron-pair arrangement. The strong repulsive force exerted by lone pairs compresses bond angles, leading to deviations from idealized geometries. Therefore, accurately predicting molecular geometry requires a thorough understanding of VSEPR theory, the role of lone pairs, and the limitations of the model. Understanding these concepts is crucial for interpreting molecular properties and reactivity. Remember that VSEPR is a useful tool, but more advanced techniques are sometimes required for complex molecules. The key lies in understanding the interplay of bonding and non-bonding electron pairs and their influence on molecular shape.
Latest Posts
Latest Posts
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonding Domains Does A Lone Pair Account For . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
