How Many Electron Domain Groups Does Water Have
Muz Play
Mar 18, 2025 · 5 min read
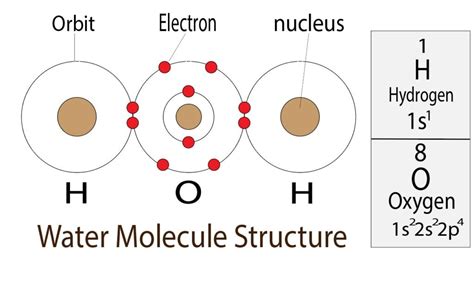
Table of Contents
How Many Electron Domain Groups Does Water Have? A Deep Dive into VSEPR Theory
Understanding the electron domain geometry of molecules is crucial in chemistry for predicting their shapes, polarity, and reactivity. This article delves deep into the electron domain groups of water (H₂O), explaining the concept in detail, exploring the VSEPR theory, and examining its implications. We'll go beyond simply stating the answer, providing a comprehensive understanding of the underlying principles.
Understanding Electron Domains
Before we dive into the specifics of water, let's define what an electron domain is. An electron domain represents a region of high electron density surrounding a central atom in a molecule. This region can be occupied by either a:
- Bonding pair: A pair of electrons shared between the central atom and another atom (forming a covalent bond).
- Lone pair: A pair of electrons that are not involved in bonding and remain associated with the central atom.
The number of electron domains surrounding a central atom dictates the molecule's electron domain geometry, which is the arrangement of these electron domains in three-dimensional space. This geometry is different from the molecular geometry, which describes the arrangement of only the atoms in the molecule. Lone pairs influence the molecular geometry because they exert a stronger repulsive force than bonding pairs.
VSEPR Theory: The Foundation of Molecular Geometry Prediction
The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone of predicting molecular geometries. It states that electron domains around a central atom will arrange themselves to minimize electrostatic repulsion, thus maximizing the distance between them. This principle is fundamental to understanding the structure of water and countless other molecules.
Applying VSEPR Theory to Water (H₂O)
Water's central atom is oxygen (O), which has six valence electrons. Each hydrogen (H) atom contributes one electron to form a covalent bond with the oxygen. This arrangement results in the following:
- Oxygen atom: Six valence electrons.
- Two hydrogen atoms: Two valence electrons (one from each).
- Total valence electrons: Eight valence electrons.
These eight electrons are arranged as four electron domains around the oxygen atom: two bonding pairs (one for each O-H bond) and two lone pairs.
Therefore, the answer to the question, "How many electron domain groups does water have?" is four.
Electron Domain Geometry vs. Molecular Geometry in Water
It's crucial to distinguish between the electron domain geometry and the molecular geometry in water.
-
Electron Domain Geometry: With four electron domains, the electron domain geometry of water is tetrahedral. This refers to the arrangement of the four electron domains around the central oxygen atom—imagining them positioned at the corners of a tetrahedron.
-
Molecular Geometry: The molecular geometry considers only the positions of the atoms. The two lone pairs on the oxygen atom significantly influence the arrangement of the hydrogen atoms. The repulsion from the lone pairs compresses the bond angle between the two O-H bonds, making the molecular geometry bent or V-shaped, rather than linear or tetrahedral.
Visualizing the Electron Domains and Molecular Geometry of Water
Imagine the oxygen atom at the center. Two electron domains are occupied by bonding pairs (O-H bonds), pushing the two hydrogen atoms away from each other. The other two electron domains are occupied by lone pairs, which repel the bonding pairs more strongly than the bonding pairs repel each other. This repulsion leads to the bent shape of the water molecule. The bond angle is approximately 104.5 degrees, slightly less than the ideal 109.5 degrees for a perfect tetrahedron. The difference arises due to the stronger repulsion of the lone pairs.
Implications of Water's Electron Domain Geometry
The four electron domains and the resulting bent molecular geometry of water have profound implications for its properties:
-
Polarity: The bent structure and the presence of lone pairs create a polar molecule. Oxygen is more electronegative than hydrogen, causing a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This polarity allows water to form hydrogen bonds, influencing its high boiling point, surface tension, and excellent solvent properties.
-
Hydrogen Bonding: The lone pairs on the oxygen atom can participate in hydrogen bonding with other water molecules or with molecules containing hydrogen atoms bonded to electronegative atoms (like nitrogen or fluorine). This hydrogen bonding significantly contributes to water's unique properties.
-
Reactivity: The electron domain geometry and molecular polarity affect water's reactivity. The lone pairs can act as Lewis bases, donating electrons to acids or other electron-deficient species.
Beyond Water: Extending VSEPR Theory
The principles discussed here for water apply to numerous other molecules. By determining the number of electron domains around a central atom, you can predict the electron domain geometry and (with consideration of lone pairs) the molecular geometry. VSEPR theory provides a powerful and relatively simple method for understanding the three-dimensional structure of molecules.
Advanced Considerations and Limitations of VSEPR
While VSEPR theory provides a useful framework for understanding molecular geometry, it has limitations:
- Oversimplification: VSEPR is a simplified model. It doesn't account for subtle effects like the size of atoms or the presence of multiple bonds.
- Limitations with Larger Molecules: Predicting geometries of larger, more complex molecules can become challenging with VSEPR alone. More sophisticated computational methods may be needed for accurate predictions.
- Exceptions: There are some molecules that do not perfectly follow the predictions of VSEPR theory due to factors not explicitly considered in the model.
Conclusion: A Foundation for Understanding Molecular Structure
Understanding the number of electron domains in a molecule is fundamental to predicting its geometry and properties. Water, with its four electron domains, serves as an excellent example to illustrate the power and limitations of VSEPR theory. Its unique bent structure, resulting from the influence of lone pairs, is directly responsible for many of its crucial properties, including its polarity and ability to form hydrogen bonds—properties that are essential for life as we know it. By grasping the underlying principles of electron domain geometry, we can unlock a deeper understanding of molecular behavior and chemical reactivity. This knowledge is indispensable for students and professionals in chemistry, biology, and related fields.
Latest Posts
Latest Posts
-
How To Calculate Expected Frequencies For Chi Square Test
Mar 18, 2025
-
What Is A Column In A Periodic Table
Mar 18, 2025
-
Final Product Of The Calvin Cycle
Mar 18, 2025
-
Is A Solution A Homogeneous Or Heterogeneous Mixture
Mar 18, 2025
-
Is S More Electronegative Than O
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electron Domain Groups Does Water Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
