Is S More Electronegative Than O
Muz Play
Mar 18, 2025 · 5 min read
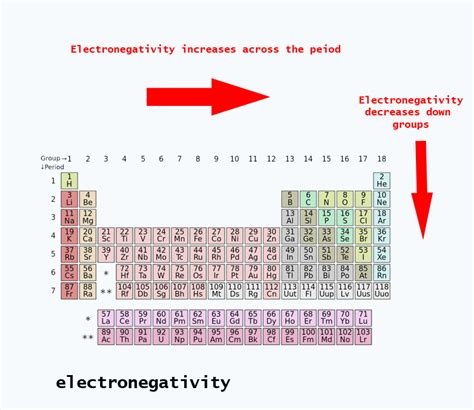
Table of Contents
Is S More Electronegative Than O? Delving into Electronegativity and its Implications
Electronegativity, a fundamental concept in chemistry, dictates the ability of an atom within a molecule to attract shared electrons towards itself. Understanding electronegativity differences is crucial for predicting molecular polarity, bond strength, and reactivity. A common point of confusion arises when comparing the electronegativity of sulfur (S) and oxygen (O). The simple answer is no, S is not more electronegative than O; O is significantly more electronegative than S. However, understanding why requires a deeper dive into the factors governing electronegativity.
Understanding Electronegativity
Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, often represented using the Pauling scale. This scale, devised by Linus Pauling, assigns fluorine (F) the highest electronegativity value of 4.0. Other elements are then assigned values relative to fluorine. Higher values indicate a greater ability to attract electrons.
Several factors influence an atom's electronegativity:
1. Nuclear Charge: The Pull of the Nucleus
The positive charge of the nucleus directly impacts electronegativity. A higher nuclear charge exerts a stronger attractive force on electrons, increasing electronegativity. Oxygen (O) has eight protons, while sulfur (S) has sixteen. While sulfur has a larger nuclear charge, this effect is counteracted by other factors.
2. Atomic Radius: Distance Matters
As you move down a group in the periodic table, atomic radius increases. This means the outermost electrons are farther from the nucleus, experiencing a weaker attraction. Sulfur is significantly larger than oxygen, placing its valence electrons further from the nucleus and reducing their attraction. This increased distance weakens the pull of the nucleus on the shared electrons.
3. Shielding Effect: Inner Electrons' Influence
Inner electrons shield the valence electrons from the full positive charge of the nucleus. Sulfur has more inner electrons than oxygen, resulting in greater shielding. These inner electrons partially block the attractive force of the nucleus on the outer electrons.
Comparing Oxygen and Sulfur
Let's directly compare oxygen and sulfur:
-
Oxygen (O): Smaller atomic radius, less shielding, relatively strong nuclear charge for its size. These factors combine to give oxygen a high electronegativity.
-
Sulfur (S): Larger atomic radius, more shielding, although a larger nuclear charge, the increased distance and shielding significantly reduce its effective nuclear charge. This leads to a lower electronegativity compared to oxygen.
Consequently, oxygen's electronegativity (3.44) is considerably higher than sulfur's (2.58) on the Pauling scale.
The Implications of Electronegativity Differences: Oxygen vs. Sulfur
The difference in electronegativity between oxygen and sulfur has significant consequences for the properties of their compounds:
1. Bond Polarity
When oxygen and another atom form a bond, the shared electrons are pulled more strongly towards the oxygen atom, creating a polar bond. The oxygen atom acquires a partial negative charge (δ-), and the other atom acquires a partial positive charge (δ+). For instance, in a water molecule (H₂O), the O-H bonds are polar, making water a polar molecule.
In sulfur-containing compounds, the polarity of the bonds is less pronounced due to sulfur's lower electronegativity. For example, the S-H bonds in hydrogen sulfide (H₂S) are less polar than the O-H bonds in water. This difference affects the overall polarity and properties of the molecules.
2. Bond Strength
Electronegativity influences bond strength. Generally, a larger electronegativity difference between two bonded atoms results in a stronger bond. However, this isn't always a straightforward relationship. While the O-H bond is more polar than the S-H bond, the O-O and S-S single bonds are relatively weak. The higher electronegativity of oxygen does not imply a stronger bond in all scenarios.
3. Oxidation States
Oxygen almost always displays a -2 oxidation state in its compounds (except in peroxides where it's -1). Its high electronegativity allows it to readily accept two electrons to achieve a stable octet. Sulfur, with its lower electronegativity, can exhibit a wider range of oxidation states, from -2 to +6. This variability is reflected in the diverse chemistry of sulfur-containing compounds.
4. Reactivity
The difference in electronegativity influences the reactivity of compounds. Oxygen's high electronegativity makes it a very reactive element, readily forming oxides with many elements. Sulfur, while less reactive than oxygen, still participates in a variety of chemical reactions. The reactivity difference is evident in the combustion process. Oxygen readily supports rapid combustion while sulfur's reaction with oxygen is considerably slower.
Beyond the Pauling Scale: Other Electronegativity Scales
While the Pauling scale is widely used, other electronegativity scales exist, such as the Mulliken scale and the Allred-Rochow scale. These scales employ different approaches and may yield slightly different values, but they all consistently show oxygen as more electronegative than sulfur. The underlying principles remain the same: nuclear charge, atomic radius, and shielding effect play dominant roles in determining electronegativity.
Practical Applications and Real-World Examples
The electronegativity difference between oxygen and sulfur has practical implications across various fields:
-
Biochemistry: Oxygen's high electronegativity plays a crucial role in the formation of hydrogen bonds in biological molecules like proteins and DNA. The polarity of O-H bonds is essential for maintaining the structure and function of these vital molecules. Sulfur, with its lower electronegativity, plays a different role, often forming disulfide bridges that contribute to protein structure.
-
Environmental Science: The reactivity of oxygen is critical in various environmental processes like combustion, oxidation, and the formation of ozone. Sulfur compounds, on the other hand, are involved in acid rain formation and other environmental issues.
-
Material Science: The properties of materials are often influenced by the electronegativity of their constituent atoms. Understanding electronegativity helps in designing and synthesizing new materials with specific properties.
Conclusion: Oxygen Reigns Supreme
In summary, the statement "S is more electronegative than O" is incorrect. Oxygen (O) is significantly more electronegative than sulfur (S). This difference stems from the interplay of nuclear charge, atomic radius, and shielding effects. The higher electronegativity of oxygen leads to significant differences in bond polarity, bond strength, oxidation states, and reactivity, with consequences spanning various scientific disciplines and impacting our understanding of the natural world. The difference is not merely an academic point; it has profound implications for the chemical behavior of countless molecules and the properties of the materials they form. The consistent observation across different electronegativity scales confirms oxygen's superior electronegativity compared to sulfur.
Latest Posts
Latest Posts
-
Shear Force Diagram Of Cantilever Beam
Mar 19, 2025
-
Mean And Variance Of Sample Mean
Mar 19, 2025
-
Predict The Product For The Reaction Shown
Mar 19, 2025
-
When Is Work Positive And Negative
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is S More Electronegative Than O . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
