Is A Solution A Homogeneous Or Heterogeneous Mixture
Muz Play
Mar 18, 2025 · 5 min read
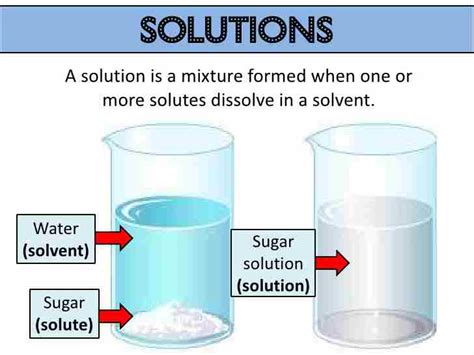
Table of Contents
Is a Solution a Homogeneous or Heterogeneous Mixture? Understanding the Nature of Solutions
The question of whether a solution is a homogeneous or heterogeneous mixture is fundamental to understanding chemistry. The answer, simply put, is that a solution is always a homogeneous mixture. However, understanding why this is true requires delving into the definitions of solutions, homogeneous mixtures, and heterogeneous mixtures, along with exploring various examples and exceptions. This comprehensive guide will explore these concepts in detail, providing a clear and concise understanding of the nature of solutions.
Defining Key Terms: Solution, Homogeneous Mixture, and Heterogeneous Mixture
Before we dive into the specifics of solutions, let's clearly define the key terms involved:
Solution: A Perfect Blend
A solution is a type of homogeneous mixture formed when one substance (the solute) dissolves in another substance (the solvent). The solute is uniformly distributed throughout the solvent at the molecular level, resulting in a single phase. This means you won't be able to visually distinguish the individual components of a solution. The properties of a solution are consistent throughout, unlike heterogeneous mixtures. Key characteristics include:
- Uniform Composition: The solute is evenly dispersed throughout the solvent.
- Single Phase: A solution exists in only one phase (solid, liquid, or gas).
- Particle Size: Solute particles are typically ions or molecules, meaning they are incredibly small.
- Filtration: Solutions cannot be separated by simple filtration.
Homogeneous Mixture: Uniformity at a Molecular Level
A homogeneous mixture is one where the composition is uniform throughout the entire mixture. This means that if you were to take a sample from any part of the mixture, its composition would be identical to a sample taken from another part. Solutions are a prime example of homogeneous mixtures, but other examples include air (a mixture of gases) and saltwater. The key defining feature is the uniform distribution of components at a microscopic level, resulting in a single, visually uniform phase.
Heterogeneous Mixture: A Visible Difference
In contrast to homogeneous mixtures, a heterogeneous mixture is one where the composition is not uniform. Different parts of the mixture have different properties and compositions. You can typically see distinct components or phases within a heterogeneous mixture. Examples include sand and water, oil and water, and a salad. The components are not evenly distributed, leading to visual variations and often the possibility of separation through simple methods like filtration or settling.
Why Solutions Are Homogeneous Mixtures: A Deep Dive
The key to understanding why solutions are always homogeneous lies in the process of dissolution. When a solute dissolves in a solvent, the solute particles (ions or molecules) are completely surrounded by solvent molecules. This process is driven by intermolecular forces – the attractive forces between molecules.
For example, when you dissolve table salt (NaCl) in water, the polar water molecules interact with the positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻) of the salt. This interaction weakens the ionic bonds holding the salt crystal together, allowing the ions to separate and become surrounded by water molecules. This process, called hydration, leads to a uniform distribution of sodium and chloride ions throughout the water, resulting in a homogeneous saltwater solution.
The size of the solute particles is also crucial. In a solution, the solute particles are extremely small – at the atomic or molecular level. This small size contributes to the uniform distribution and inability to visually distinguish the components. This is in stark contrast to heterogeneous mixtures, where the components may be visibly distinct due to larger particle sizes.
Examples of Solutions and Their Homogeneous Nature
Let's explore some common examples to further illustrate the homogeneous nature of solutions:
- Sugar in Water: When you dissolve sugar in water, the sugar molecules distribute uniformly throughout the water, creating a sweet, homogenous solution. You can't see individual sugar molecules; the solution appears clear and consistent.
- Salt in Water (as discussed above): The ions of salt disperse evenly within the water molecules, making it a homogeneous mixture.
- Air: Although seemingly simple, air is a solution of various gases (primarily nitrogen and oxygen), mixed homogeneously.
- Brass: This alloy is a solid solution of copper and zinc, where the atoms of both metals are uniformly distributed throughout the structure.
- Seawater: This is a solution of various salts and minerals dissolved in water. The dissolved components are distributed uniformly, making it homogeneous.
Apparent Exceptions: Addressing the Gray Areas
While most solutions are clearly homogeneous, some situations might appear to contradict this. However, upon closer inspection, these apparent exceptions often reveal underlying principles that reinforce the definition of a solution.
- Supersaturated Solutions: These solutions contain more solute than they can normally hold at a given temperature. While appearing homogeneous initially, they are metastable and can easily precipitate out excess solute, revealing their inherent homogeneity. The initial homogeneous distribution before precipitation confirms the underlying principle.
- Colloids: These mixtures are sometimes confused with solutions, but they are distinct. Colloids contain particles larger than those in a solution (but smaller than those in a suspension), leading to the Tyndall effect (scattering of light). While they may appear homogeneous at first glance, they are technically heterogeneous due to the larger particle size and their ability to scatter light. Milk and fog are examples of colloids.
- Suspensions: These are clearly heterogeneous mixtures. They consist of larger particles that will settle out over time. Sand in water is a classic example. The distinct separation of components makes them easily distinguishable from solutions.
Conclusion: Solutions: The Epitome of Homogeneous Mixtures
In conclusion, a solution is always a homogeneous mixture. The uniform distribution of solute particles at the molecular level is the defining characteristic that sets solutions apart. While some situations might appear to blur the lines, a thorough understanding of the definitions and processes involved reinforces the fundamental truth that true solutions are, without exception, homogeneous mixtures. Understanding this distinction is crucial for comprehending various chemical and physical processes and further exploring the fascinating world of mixtures and solutions. This knowledge is fundamental in various scientific disciplines, from chemistry and biology to materials science and environmental studies.
Latest Posts
Latest Posts
-
During Glycolysis Glucose Is Broken Down Into
Mar 19, 2025
-
How To Identify An Acid From Its Chemical Formula
Mar 19, 2025
-
Investigation Dna Proteins And Sickle Cell Answer Key
Mar 19, 2025
-
Buffer Region On A Titration Curve
Mar 19, 2025
-
Milk Of Magnesia Is Acidic Or Basic
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is A Solution A Homogeneous Or Heterogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
