Milk Of Magnesia Is Acidic Or Basic
Muz Play
Mar 19, 2025 · 5 min read
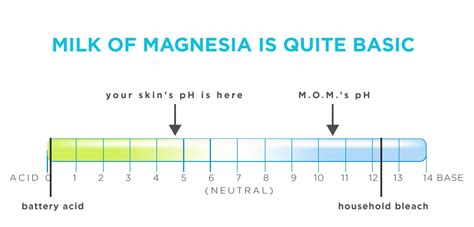
Table of Contents
Is Milk of Magnesia Acidic or Basic? Understanding pH and Antacids
Milk of magnesia, a common over-the-counter remedy for constipation and heartburn, often sparks the question: is milk of magnesia acidic or basic? The answer is crucial for understanding how it works and its potential interactions with other medications. This comprehensive guide delves into the chemical composition of milk of magnesia, explains its pH level, explores its mechanism of action, and discusses its uses and potential side effects.
Understanding pH and the pH Scale
Before we dive into the specifics of milk of magnesia, let's establish a basic understanding of pH. The pH scale measures how acidic or basic (alkaline) a substance is. It ranges from 0 to 14, with:
- 0-6: Acidic substances (e.g., lemon juice, stomach acid)
- 7: Neutral (e.g., pure water)
- 8-14: Basic or alkaline substances (e.g., baking soda, ammonia)
The scale is logarithmic, meaning each whole number change represents a tenfold change in acidity or basicity. For example, a substance with a pH of 3 is ten times more acidic than a substance with a pH of 4.
The Chemical Composition of Milk of Magnesia
Milk of magnesia, also known as magnesium hydroxide, is a suspension of magnesium hydroxide in water. Its chemical formula is Mg(OH)₂. This is the key to understanding its pH. Magnesium hydroxide is a weak base. When dissolved in water, it partially dissociates into magnesium ions (Mg²⁺) and hydroxide ions (OH⁻). These hydroxide ions are what contribute to the alkaline nature of milk of magnesia.
Why is Magnesium Hydroxide a Base?
The presence of hydroxide ions (OH⁻) is the defining characteristic of a base. Hydroxide ions readily accept protons (H⁺), which is the fundamental characteristic of a base in the Brønsted-Lowry acid-base theory. This ability to accept protons is what neutralizes acids.
The pH of Milk of Magnesia: Definitely Basic!
Milk of magnesia has a pH significantly greater than 7, confirming its basic nature. The exact pH can vary slightly depending on the concentration of the suspension, but it generally falls within the range of 10-11. This high pH is crucial for its effectiveness as an antacid and laxative.
How Milk of Magnesia Works: Neutralization and Osmosis
Milk of magnesia's basic nature is directly related to its mechanisms of action:
1. Antacid Action: Neutralizing Stomach Acid
When ingested, milk of magnesia's hydroxide ions react with the hydrochloric acid (HCl) present in the stomach. This is a neutralization reaction, producing water and magnesium chloride:
Mg(OH)₂ + 2HCl → 2H₂O + MgCl₂
This reaction reduces the acidity in the stomach, relieving symptoms of heartburn, acid indigestion, and sour stomach.
2. Laxative Action: Osmotic Effect
Milk of magnesia also acts as a laxative. Its high pH and magnesium content contribute to this effect. The magnesium ions draw water into the intestines through osmosis, softening the stool and increasing bowel movements. This increased water content makes the stool easier to pass, relieving constipation.
Milk of Magnesia vs. Other Antacids: A Comparison
Many over-the-counter antacids are available, each with a different chemical composition and mechanism of action. Understanding these differences is crucial for selecting the appropriate medication:
- Calcium carbonate (Tums): A weak base that neutralizes stomach acid. It's effective but can cause constipation in some individuals.
- Sodium bicarbonate (baking soda): A strong base that neutralizes stomach acid rapidly. However, it can lead to sodium retention and is not recommended for regular use.
- Aluminum hydroxide (Amphogel): A weak base that neutralizes stomach acid and can also bind to phosphates, making it useful for people with kidney disease. However, it can cause constipation.
Milk of Magnesia offers a unique combination of antacid and laxative properties, setting it apart from other options. Its effectiveness and side effect profile should be considered when choosing an antacid.
Uses and Potential Side Effects of Milk of Magnesia
Milk of Magnesia's primary uses include:
- Relief of constipation: Its osmotic effect effectively softens stool and promotes bowel movements.
- Relief of heartburn and acid indigestion: Its neutralizing effect on stomach acid reduces discomfort.
However, like all medications, milk of magnesia can cause side effects, especially with overuse or misuse:
- Diarrhea: Excessive use can lead to diarrhea due to its osmotic effect.
- Electrolyte imbalance: High doses can interfere with electrolyte balance, particularly magnesium levels. This is a particular concern for individuals with kidney problems.
- Nausea and vomiting: These are less common side effects but can occur in sensitive individuals.
When to Consult a Doctor
While generally safe for occasional use, it's important to consult a doctor before using milk of magnesia regularly or for extended periods. This is especially important if you:
- Have kidney disease
- Are pregnant or breastfeeding
- Are taking other medications
- Have a history of electrolyte imbalances
- Experience persistent or severe digestive problems
Your doctor can assess your individual needs and recommend the most appropriate treatment. Self-treating can sometimes mask underlying medical conditions, and professional guidance is always advisable.
Conclusion: Milk of Magnesia: A Basic Solution for Common Ailments
Milk of magnesia is definitively basic, boasting a pH significantly above 7. This basic nature is fundamental to its effectiveness as both an antacid and a laxative. Its ability to neutralize stomach acid and draw water into the intestines makes it a valuable over-the-counter remedy for occasional constipation and heartburn. However, understanding its potential side effects and consulting a doctor when necessary is crucial for safe and effective use. Remember, while readily available, it's not a solution for every digestive problem and should be used judiciously. Always read the label carefully and follow the recommended dosage instructions. Responsible self-care involves understanding the medications you use and seeking professional advice when needed.
Latest Posts
Latest Posts
-
Is H2o An Acid Or Base
Mar 19, 2025
-
What Are The Membrane Bound Organelles
Mar 19, 2025
-
What Does A Penetration Pricing Demand Curve Look Like
Mar 19, 2025
-
How To Calculate Heat Of Reaction In Kj Mol
Mar 19, 2025
-
What Is Zone Of Inhibition In Microbiology
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Milk Of Magnesia Is Acidic Or Basic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
