How Many Electrons Can An F-orbital Hold
Muz Play
Mar 15, 2025 · 5 min read
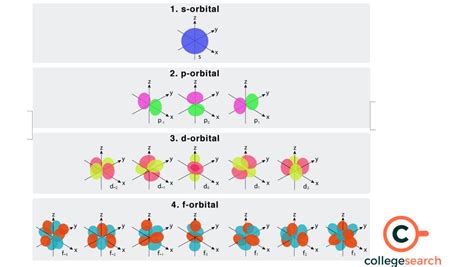
Table of Contents
How Many Electrons Can an F-Orbital Hold? A Deep Dive into Atomic Structure
Understanding the electron capacity of orbitals is fundamental to grasping the intricacies of atomic structure and the periodic table. While s and p orbitals are relatively straightforward, the f-orbital presents a slightly more complex scenario. This comprehensive guide will delve into the answer to the question: How many electrons can an f-orbital hold? We'll explore the quantum numbers that define orbitals, the spatial arrangement of f-orbitals, and the implications of their electron capacity for chemical properties.
Understanding Atomic Orbitals and Quantum Numbers
Before addressing the f-orbital specifically, let's establish a foundational understanding of atomic orbitals. Orbitals are regions of space within an atom where there's a high probability of finding an electron. Their shapes and energy levels are defined by a set of quantum numbers:
1. Principal Quantum Number (n)
This number represents the energy level of the electron and the average distance of the electron from the nucleus. It's always a positive integer (n = 1, 2, 3, ...). Higher values of n indicate higher energy levels and larger orbitals.
2. Azimuthal Quantum Number (l)
This quantum number describes the shape of the orbital and its angular momentum. It can take integer values from 0 to n - 1. Each value of l corresponds to a specific orbital type:
- l = 0: s-orbital (spherical)
- l = 1: p-orbital (dumbbell-shaped)
- l = 2: d-orbital (more complex shapes)
- l = 3: f-orbital (even more complex shapes)
3. Magnetic Quantum Number (ml)
This quantum number specifies the orientation of the orbital in space. It can take integer values from -l to +l, including 0. For example:
- For an s-orbital (l=0), ml = 0 (one orientation).
- For a p-orbital (l=1), ml = -1, 0, +1 (three orientations).
- For a d-orbital (l=2), ml = -2, -1, 0, +1, +2 (five orientations).
4. Spin Quantum Number (ms)
This quantum number describes the intrinsic angular momentum of the electron, often referred to as its "spin." It can have only two values: +1/2 (spin up) or -1/2 (spin down). This is crucial because it dictates that each orbital can hold a maximum of two electrons, with opposite spins (Pauli Exclusion Principle).
The F-Orbital: Shape and Orientation
The f-orbital, characterized by l = 3, possesses a set of seven orbitals due to the seven possible values of the magnetic quantum number (ml = -3, -2, -1, 0, +1, +2, +3). These orbitals have complex, three-dimensional shapes that are difficult to visualize easily. Unlike the simpler s, p, and even d orbitals, the f orbitals lack easily describable shapes. They are characterized by regions of high electron probability distributed in intricate patterns around the nucleus.
The complex shapes arise from the interplay of the different magnetic quantum numbers. The seven f orbitals possess different orientations in space, contributing to the overall electronic structure of the atom. Visual representations often use lobes and nodal planes to depict the regions where electron density is high and low, respectively. However, these are simplified illustrations; the actual electron distribution is described by the wave function, which is a more mathematically complex representation.
The Electron Capacity of an F-Orbital
Now, let's get to the heart of the matter. Each individual f-orbital can hold a maximum of two electrons, following the Pauli Exclusion Principle. Since there are seven f-orbitals in a given subshell, a complete f-subshell can accommodate a total of fourteen electrons (7 orbitals x 2 electrons/orbital = 14 electrons).
This has significant implications for the elements in the periodic table. The lanthanides and actinides series, located at the bottom of the periodic table, are characterized by the filling of their 4f and 5f orbitals, respectively. This is what gives rise to their unique chemical properties and complex electronic configurations.
Implications for Chemical Properties
The fourteen electrons in a fully filled f-subshell significantly impact the chemical behavior of the elements. A full f-subshell results in a relatively stable electronic configuration, contributing to similar chemical properties within the lanthanide and actinide series. However, subtle differences in chemical properties do exist, often attributed to the lanthanide contraction and other factors influencing electron-electron interactions.
The shielding effect of the f-electrons also plays a crucial role. The f-electrons, while relatively poorly shielding, do contribute to shielding the outer electrons from the nucleus. This influences the effective nuclear charge felt by the valence electrons, affecting various chemical properties like ionization energy, electronegativity, and atomic radius.
Beyond the Basics: Electron Configuration and Hund's Rule
Understanding the electron capacity of the f-orbital is just one aspect of a broader understanding of electron configuration. Hund's rule plays a crucial role in determining how electrons populate the orbitals within a subshell. Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This means that before pairing electrons within an f-orbital, each of the seven f-orbitals will first contain one electron each, all with parallel spins.
This filling process of f-orbitals influences the magnetic properties of the elements. The presence of unpaired electrons leads to paramagnetism, meaning the atoms will be attracted to an external magnetic field. However, when the f-subshell is completely filled, the electrons are paired, resulting in diamagnetism, where the atom is slightly repelled by an external magnetic field.
Conclusion: The Significance of F-Orbital Electron Capacity
The capacity of an f-orbital to hold up to fourteen electrons is a key concept in atomic structure and chemistry. This understanding is essential for comprehending the properties and behavior of the lanthanides and actinides, elements with significant applications in various fields, including catalysis, materials science, and nuclear technology. The intricate shapes and energy levels of the f-orbitals, coupled with the principles of electron configuration and quantum mechanics, contribute to the complexity and fascinating behavior of these elements in the periodic table. By mastering this fundamental concept, one gains a deeper appreciation for the underlying principles governing the structure and reactivity of matter. The information provided here serves as a foundation for further exploration of advanced topics in quantum chemistry and the fascinating world of atomic structure.
Latest Posts
Latest Posts
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can An F-orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
