How Many Electrons Does The D Orbital Hold
Muz Play
Mar 17, 2025 · 6 min read
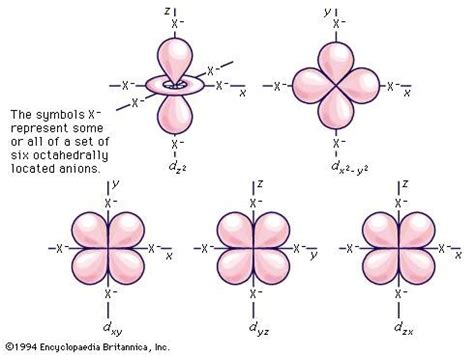
Table of Contents
How Many Electrons Does the D Orbital Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to grasping the principles of chemistry and physics. A key component of this understanding lies in comprehending atomic orbitals, particularly the d orbital. This article delves into the intricacies of the d orbital, exploring its shape, energy levels, electron capacity, and its significance in chemical bonding and properties of elements.
Unveiling the Mystery of the D Orbital
Before we dive into the electron capacity of the d orbital, let's establish a foundational understanding of atomic orbitals. According to the quantum mechanical model of the atom, electrons don't orbit the nucleus in fixed paths like planets around a star. Instead, they exist in regions of space called atomic orbitals, which represent the probability of finding an electron at a given location.
These orbitals are characterized by specific quantum numbers:
-
Principal Quantum Number (n): Determines the energy level and size of the orbital. It's a positive integer (n = 1, 2, 3...). Higher 'n' values indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): Specifies the shape of the orbital and its angular momentum. It can take integer values from 0 to n-1. l=0 corresponds to an s orbital, l=1 to a p orbital, l=2 to a d orbital, and l=3 to an f orbital.
-
Magnetic Quantum Number (ml): Describes the orientation of the orbital in space. It can take integer values from -l to +l, including 0.
-
Spin Quantum Number (ms): Represents the intrinsic angular momentum of the electron, which is either +1/2 or -1/2 (spin up or spin down).
The d orbital, with l=2, is where things get interesting. Unlike the simpler spherical s orbitals and dumbbell-shaped p orbitals, the d orbitals exhibit more complex shapes. There are five d orbitals in each energy level (n ≥ 3), each with a unique spatial orientation:
The Five d Orbitals: A Closer Look
The five d orbitals are often labeled as d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>, d<sub>x²-y²</sub>, and d<sub>z²</sub>. These labels reflect the orientation of the electron density within the orbitals relative to the x, y, and z axes in three-dimensional space. While the precise mathematical descriptions are complex, visualizing their general shapes is crucial for understanding their properties.
-
d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>: These orbitals have electron density concentrated between the axes.
-
d<sub>x²-y²</sub>: This orbital has electron density concentrated along the x and y axes, with nodes (regions of zero electron density) along the diagonals.
-
d<sub>z²</sub>: This orbital has a unique shape with a dumbbell along the z-axis and a toroidal (doughnut-shaped) distribution in the xy plane.
The Crucial Question: How Many Electrons Can a D Orbital Hold?
Each atomic orbital, regardless of its type (s, p, d, f), can hold a maximum of two electrons. This is a consequence of the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers. Since each electron in an orbital has its own spin quantum number (either +1/2 or -1/2), an orbital can accommodate a maximum of two electrons with opposite spins.
Therefore, since there are five d orbitals in a given energy level (n ≥ 3), a complete set of d orbitals can hold a maximum of 2 electrons/orbital * 5 orbitals = 10 electrons.
The Significance of d Orbitals in Chemistry
The d orbitals play a crucial role in the chemistry of transition metals. Transition metals are elements located in the d-block of the periodic table, meaning their outermost electrons occupy d orbitals. The involvement of d electrons in chemical bonding accounts for many unique properties of transition metals, including:
-
Variable Oxidation States: Transition metals readily exhibit multiple oxidation states due to the relatively small energy difference between their (n-1)d and ns orbitals. This allows them to lose varying numbers of electrons to form ions.
-
Colored Compounds: The partially filled d orbitals of transition metal ions allow for the absorption of specific wavelengths of light, resulting in the characteristic colors observed in many transition metal compounds.
-
Catalytic Activity: The ability of transition metals to exist in multiple oxidation states and to form complexes with various ligands makes them effective catalysts in many chemical reactions.
-
Magnetic Properties: The presence of unpaired electrons in d orbitals contributes to the paramagnetic behavior (attraction to a magnetic field) of many transition metal compounds.
Filling the d Orbitals: Hund's Rule and Electron Configuration
When filling the d orbitals with electrons, Hund's Rule comes into play. Hund's Rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and results in a more stable configuration.
For example, consider chromium (Cr), which has an electron configuration of [Ar] 3d<sup>5</sup> 4s<sup>1</sup>. While one might expect a 3d<sup>4</sup> 4s<sup>2</sup> configuration, the half-filled d subshell (five electrons, each in a separate orbital) and half-filled s subshell provide extra stability. This illustrates the complex interplay of energy levels and electron-electron interactions in determining electron configurations.
Beyond the Basics: Advanced Concepts
The simple picture of d orbitals presented here is a simplification. In reality, the shapes and energies of d orbitals can be significantly influenced by the surrounding atoms in a molecule or crystal. This leads to concepts like:
-
Crystal Field Theory: Explains the splitting of d orbital energies in the presence of ligands (atoms or molecules bound to a central metal ion). This splitting is crucial in understanding the color and magnetic properties of transition metal complexes.
-
Ligand Field Theory: A more sophisticated approach that combines molecular orbital theory with crystal field theory to provide a more accurate description of the electronic structure of coordination compounds.
Conclusion: A Foundation for Deeper Understanding
The d orbital, with its capacity to hold up to 10 electrons, is a cornerstone of atomic structure and plays a pivotal role in determining the chemical and physical properties of elements, especially transition metals. Understanding its shape, electron capacity, and influence on bonding is essential for mastering concepts in inorganic chemistry, materials science, and many other related fields. This article provides a robust foundation for further exploration of these fascinating aspects of atomic structure and chemical bonding. The intricacies of the d orbital demonstrate the beauty and complexity of the quantum world and its profound influence on the macroscopic world we inhabit. Further research into crystal field theory and ligand field theory will unlock deeper insights into the behavior of transition metal compounds and their numerous applications.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does The D Orbital Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
