How Many Hydrogen Bonds Can Water Form
Muz Play
Mar 22, 2025 · 6 min read
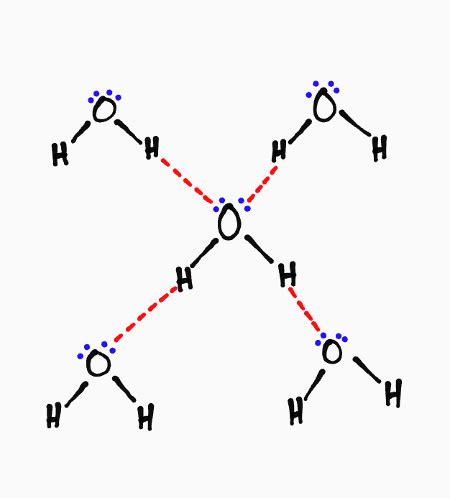
Table of Contents
How Many Hydrogen Bonds Can Water Form? Exploring the Tetrahedral Dance of H₂O
Water, the elixir of life, is a remarkably simple molecule: two hydrogen atoms covalently bonded to a single oxygen atom (H₂O). Yet, this seemingly uncomplicated structure underpins a complexity of properties that makes life on Earth possible. Central to this complexity is the ability of water molecules to form hydrogen bonds. But how many hydrogen bonds can a single water molecule actually form? The answer, while seemingly straightforward, reveals a fascinating interplay of geometry and energetics.
Understanding Hydrogen Bonds: A Gentle Introduction
Before diving into the specifics of water's hydrogen bonding capabilities, let's establish a fundamental understanding of what hydrogen bonds are. A hydrogen bond is a special type of dipole-dipole attraction between molecules, not a covalent bond. It occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. This electronegativity difference creates a partial positive charge (δ+) on the hydrogen and a partial negative charge (δ-) on the electronegative atom, leading to an electrostatic attraction.
In water, the oxygen atom is significantly more electronegative than the hydrogen atoms. This creates a polar molecule with a slightly negative oxygen and slightly positive hydrogens. This polarity is the key to water's extensive hydrogen bonding network.
The Tetrahedral Arrangement: The Key to Water's Bonding Capacity
The geometry of the water molecule plays a crucial role in determining how many hydrogen bonds it can form. The oxygen atom sits at the center of a bent molecule, with the two hydrogen atoms forming an angle of approximately 104.5 degrees. This bent shape, along with the presence of two lone pairs of electrons on the oxygen atom, leads to a tetrahedral arrangement of electron density around the oxygen.
This tetrahedral arrangement is paramount. Think of it as a three-dimensional structure with the oxygen atom at the center and the two hydrogen atoms and two lone pairs of electrons occupying the four corners of a tetrahedron. Each of these four corners can potentially participate in a hydrogen bond.
Maximum Potential: Four Hydrogen Bonds
Therefore, theoretically, a single water molecule can form a maximum of four hydrogen bonds. Two of these bonds are formed using its two hydrogen atoms, each donating a proton to a lone pair of electrons on separate oxygen atoms in neighboring water molecules. The other two bonds are formed using the two lone pairs of electrons on the oxygen atom, accepting protons from hydrogen atoms in other water molecules.
This ability to form four hydrogen bonds is the cornerstone of many of water's unique properties, such as its high boiling point, high surface tension, high specific heat capacity, and its ability to act as a universal solvent. The extensive network of hydrogen bonds creates strong intermolecular forces, requiring more energy to overcome these attractions and thus resulting in higher boiling points and greater cohesive forces.
The Reality of Dynamic Equilibrium: Not Always Four
While a water molecule can form four hydrogen bonds, it doesn't always do so. The actual number of hydrogen bonds formed by a water molecule at any given time depends on several factors including:
-
Temperature: At higher temperatures, the kinetic energy of the molecules increases, making it more difficult for hydrogen bonds to form and maintain. Consequently, the average number of hydrogen bonds per water molecule decreases at higher temperatures.
-
Pressure: Increased pressure can force water molecules closer together, favoring hydrogen bond formation.
-
Presence of other molecules: The presence of other substances dissolved in water can interfere with hydrogen bonding, either by competing for hydrogen bonds or by disrupting the water's hydrogen bond network. For example, adding electrolytes can alter the number and strength of hydrogen bonds.
-
Phase of water: The state of water (solid, liquid, or gas) significantly influences hydrogen bonding. In ice, each water molecule forms four hydrogen bonds, resulting in a highly ordered crystalline structure. In liquid water, the situation is far more dynamic, with a constantly fluctuating network of hydrogen bonds, constantly breaking and reforming. In gaseous water, hydrogen bonding is minimal.
Therefore, it's more accurate to say that a water molecule has the potential to form four hydrogen bonds rather than always forming four. The average number of hydrogen bonds per water molecule in liquid water at room temperature is closer to 3.4, reflecting the dynamic nature of this process.
The Importance of Hydrogen Bonding in Biological Systems
The ability of water to form hydrogen bonds is fundamentally important for life as we know it. Many biological processes rely on the unique properties of water that arise from this extensive hydrogen bonding network. Examples include:
-
Protein folding: Hydrogen bonds play a crucial role in stabilizing the three-dimensional structures of proteins. The precise folding of a protein dictates its function, and hydrogen bonds contribute significantly to this intricate folding process.
-
DNA structure: The double helix structure of DNA is held together by hydrogen bonds between complementary base pairs (adenine-thymine and guanine-cytosine). The specificity of these hydrogen bonds is vital for accurate DNA replication and transcription.
-
Enzyme-substrate interactions: Hydrogen bonds are often involved in the binding of enzymes to their substrates, facilitating catalytic activity. The precise positioning of substrates within the enzyme's active site is often mediated by hydrogen bonds.
-
Cell membrane structure: The hydrophilic (water-loving) and hydrophobic (water-fearing) interactions within cell membranes are partly influenced by hydrogen bonding between water molecules and the polar components of the membrane.
-
Solvent properties: Water's ability to dissolve many polar and ionic compounds is largely due to its ability to form hydrogen bonds with these solutes. This allows for the transport and interaction of various molecules within biological systems.
Further Considerations: Beyond the Basics
The discussion above focuses primarily on the hydrogen bonds formed between water molecules. However, water molecules can also form hydrogen bonds with other molecules containing electronegative atoms such as oxygen, nitrogen, and fluorine. This ability to form hydrogen bonds with a wide range of molecules contributes to water's remarkable solvent properties and its crucial role in biological systems. The strength of these bonds can vary depending on the nature of the interacting molecules and their environment.
The study of hydrogen bonding in water is an active area of research, with ongoing investigations into its precise dynamics and its implications for various natural phenomena and technological applications. Advanced computational techniques are frequently employed to model and simulate the complex interactions within water's hydrogen bond network.
Conclusion: A Dynamic and Vital Interaction
In conclusion, while a water molecule can potentially form four hydrogen bonds due to its tetrahedral arrangement of electron density, it doesn't always do so. The actual number of bonds formed is a dynamic equilibrium, influenced by temperature, pressure, and the presence of other molecules. This ability to form hydrogen bonds, with its associated implications for water's unique properties, is fundamentally important for the existence and functioning of life on Earth. The intricate dance of hydrogen bonds within water is a continuous and vital process shaping our world.
Latest Posts
Latest Posts
-
How Was The Modern Periodic Table Arranged
Mar 22, 2025
-
Openings That Allow For Gas Exchange
Mar 22, 2025
-
Determinants Of Price Elasticity Of Supply
Mar 22, 2025
-
What Is Stronger Ionic Or Covalent Bonds
Mar 22, 2025
-
Solve The System Of Linear Equations Algebraically
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Hydrogen Bonds Can Water Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
