How Was The Modern Periodic Table Arranged
Muz Play
Mar 22, 2025 · 6 min read
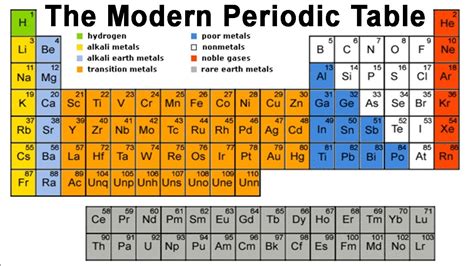
Table of Contents
How Was the Modern Periodic Table Arranged? A Journey Through Chemical History
The modern periodic table, a cornerstone of chemistry, isn't a sudden invention but the culmination of centuries of scientific inquiry and ingenious organization. Understanding its arrangement requires a journey through the history of chemistry, exploring the discoveries and insights that shaped our understanding of elements and their properties. This journey reveals not only how the table was arranged but also why it's such a powerful tool for predicting and understanding chemical behavior.
Early Attempts at Classification: The Search for Order
Before the modern periodic table, chemists grappled with a growing list of elements, each with unique properties. The need for an organizing principle became increasingly apparent. Early attempts focused on simple classifications based on observable characteristics:
Metals vs. Non-metals:
This was one of the earliest distinctions. Metals, typically shiny, conductive, and malleable, were grouped separately from non-metals, which often exhibited contrasting properties. However, this binary classification proved inadequate as the number of known elements increased. Many elements displayed properties intermediate between these two categories.
Triads:
Johann Wolfgang Döbereiner, in the 1800s, noticed that certain groups of three elements (triads) shared similar properties and that the atomic weight of the middle element was approximately the average of the other two. For example, lithium, sodium, and potassium formed a triad, showing similar reactivity. This offered a glimpse into patterns but couldn't account for all elements.
Octaves:
John Newlands, in 1864, proposed the "Law of Octaves," observing that when elements were arranged in order of increasing atomic weight, similar properties seemed to reappear every eighth element, much like musical octaves. While insightful, this law also failed for heavier elements, where the pattern broke down.
Mendeleev's Breakthrough: The Periodic Law
Dmitri Mendeleev's work in 1869 marked a turning point. He didn't just arrange elements based on atomic weight; he prioritized periodically recurring properties. This crucial distinction led to the development of the periodic law, which states that the properties of elements are a periodic function of their atomic weights.
Mendeleev's Table's Key Features:
- Arrangement by Atomic Weight: Elements were initially arranged in ascending order of their atomic weights, a relatively well-established property at the time.
- Emphasis on Recurring Properties: Mendeleev astutely placed elements in columns (groups) based on their shared chemical and physical properties, even if it meant leaving gaps. This was a radical departure from previous attempts.
- Prediction of Missing Elements: The most impressive aspect was Mendeleev's audacious prediction of undiscovered elements. He left gaps in his table, predicting their properties based on the surrounding elements. This bold prediction proved remarkably accurate when these elements were subsequently discovered. For example, he predicted the existence and properties of gallium (eka-aluminum), germanium (eka-silicon), and scandium (eka-boron). These accurate predictions provided strong evidence supporting his periodic law.
- Modifications Based on New Discoveries: Mendeleev's table was not static. He readily adjusted it as new elements were discovered and atomic weights were refined. This flexibility demonstrated the table's adaptability and power.
The Role of Atomic Number: Moseley's Contribution
While Mendeleev's table was a revolutionary achievement, some anomalies remained. Certain elements seemed out of order based solely on atomic weight. Henry Moseley's work in the early 20th century resolved this issue. Through his experiments with X-ray spectroscopy, Moseley discovered that the elements' properties were better correlated with their atomic number (the number of protons in the nucleus) than their atomic weight.
Atomic Number as the Fundamental Organizing Principle:
Moseley's research showed that atomic number, a fundamental property reflecting the element's nuclear charge, provided a more accurate and consistent basis for arranging elements in the periodic table. This led to the modern version of the periodic table, where elements are arranged strictly by increasing atomic number. This resolved several inconsistencies and solidified the table's structural foundation.
The Modern Periodic Table: Structure and Organization
The modern periodic table is a marvel of organization, reflecting the underlying structure of atoms and their interactions. Its arrangement reveals profound relationships between elements:
Periods (Rows):
Each row represents a period, corresponding to the filling of a principal energy level (electron shell) in an atom. Elements in the same period have the same number of electron shells.
Groups (Columns):
Each column represents a group or family, containing elements with similar chemical properties. This similarity stems from their having the same number of valence electrons (electrons in the outermost shell), which dictates their reactivity.
Blocks: s, p, d, and f Blocks:
The periodic table is further subdivided into blocks based on the subshells being filled:
- s-block: Elements in Groups 1 and 2, characterized by filling of the s subshell. These include alkali metals and alkaline earth metals.
- p-block: Elements in Groups 13 to 18, characterized by filling of the p subshell. This block includes a wide range of elements, from non-metals to metalloids to some metals.
- d-block: Elements in Groups 3 to 12, characterized by filling of the d subshell. These elements are known as transition metals, known for their diverse and often colorful compounds.
- f-block: Lanthanides and actinides, placed separately at the bottom of the table, characterized by filling of the f subshell. These elements exhibit similar properties within their respective series.
Predicting Properties and Trends: The Power of the Periodic Table
The arrangement of the periodic table isn't just an organizational tool; it's a powerful predictive instrument. The table allows chemists to:
Predict Chemical Reactivity:
Elements within the same group exhibit similar reactivity due to their identical number of valence electrons. This allows chemists to anticipate how an element might behave in a chemical reaction based on its position in the table.
Predict Physical Properties:
Trends in physical properties, such as atomic radius, ionization energy, electronegativity, and melting point, are also readily observed across periods and groups. These trends are directly related to the electron configuration and nuclear charge of the elements, allowing for reasonable estimations of their physical properties.
Understand Chemical Bonding:
The periodic table provides insight into the types of chemical bonds that elements are likely to form. For example, the position of an element dictates its tendency to gain, lose, or share electrons, influencing the formation of ionic, covalent, or metallic bonds.
Conclusion: A Continuing Legacy
The modern periodic table is a testament to the power of scientific inquiry and the beauty of underlying natural order. From early attempts at simple classification to Mendeleev's revolutionary approach and Moseley's crucial contribution, the story of the periodic table is a narrative of incremental progress and insightful leaps. Its arrangement, firmly grounded in atomic structure and periodic properties, continues to be a fundamental tool for understanding and predicting chemical behavior, remaining an indispensable resource for chemists worldwide. The table's enduring legacy lies not only in its organizational prowess but also in its remarkable ability to predict and explain the vast diversity of chemical phenomena, making it a truly essential instrument in the world of chemistry.
Latest Posts
Latest Posts
-
How Do You Find The Domain Of A Function Algebraically
Mar 23, 2025
-
Pain Heat And Cold Are Detected By
Mar 23, 2025
-
What Statements Are Always True About Limiting Reactants
Mar 23, 2025
-
Atoms Of The Same Element Have The Same Number Of
Mar 23, 2025
-
Individuals Considered Members Of The Same Social Category Or Group
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Was The Modern Periodic Table Arranged . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
