What Statements Are Always True About Limiting Reactants
Muz Play
Mar 23, 2025 · 6 min read
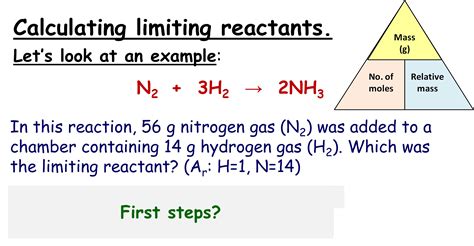
Table of Contents
What Statements Are Always True About Limiting Reactants?
Understanding limiting reactants is crucial in stoichiometry, the cornerstone of many chemical calculations. While the concept seems straightforward, nuances often lead to confusion. This comprehensive guide delves into the definitive truths about limiting reactants, clarifying common misconceptions and providing a robust understanding for students and professionals alike.
Defining the Limiting Reactant
Before exploring statements always true about limiting reactants, let's firmly establish the definition. The limiting reactant (or limiting reagent) is the reactant that is completely consumed first in a chemical reaction, thus limiting the amount of product that can be formed. It's the reactant that determines the maximum yield of the reaction. Once the limiting reactant is used up, the reaction stops, regardless of how much of the other reactants remains.
The reactants that are left over after the limiting reactant is completely consumed are called excess reactants.
Statements Always True About Limiting Reactants
Several statements are invariably true regarding limiting reactants:
1. The Limiting Reactant Determines the Maximum Yield
This is the foundational truth. No matter how much of the excess reactants are present, the amount of product formed is directly proportional to the amount of the limiting reactant available. If you double the amount of the limiting reactant, you (ideally) double the amount of product. Conversely, if you have less limiting reactant, you get less product. This is a cornerstone of stoichiometric calculations.
Example: Consider the reaction: 2H₂ + O₂ → 2H₂O. If you have 4 moles of H₂ and 1 mole of O₂, O₂ is the limiting reactant because the stoichiometry shows that 2 moles of H₂ are needed for every 1 mole of O₂. Even though you have excess H₂, you can only produce a maximum of 2 moles of H₂O (because you only have 1 mole of O₂ to react).
2. The Limiting Reactant is Completely Consumed
By definition, the limiting reactant is entirely used up during the reaction. No molecules of the limiting reactant will remain at the end of the reaction (assuming the reaction goes to completion). This complete consumption is what defines its "limiting" nature – it limits the reaction's progress.
3. The Amount of Product Formed is Directly Proportional to the Moles of the Limiting Reactant
The mole ratio between the limiting reactant and the product(s) dictates the amount of product formed. This is determined by the balanced chemical equation. This proportionality holds true under ideal reaction conditions (100% yield). In real-world scenarios, the actual yield might be less due to side reactions, incomplete reactions, or losses during product isolation.
4. The Identity of the Limiting Reactant Depends on the Initial Molar Ratios of Reactants
The limiting reactant isn't an intrinsic property of a reactant; it depends entirely on the initial quantities of all the reactants present. If you change the initial amounts of the reactants, you can change which reactant becomes limiting. A reactant that is limiting in one scenario might be in excess in another.
5. Calculating the Limiting Reactant Requires a Balanced Chemical Equation
A balanced chemical equation provides the crucial mole ratios between reactants and products. Without this balanced equation, you cannot accurately determine the limiting reactant. The coefficients in the balanced equation are essential for stoichiometric calculations. These coefficients show the relative amounts of each substance involved in the reaction.
6. Multiple Limiting Reactants are Possible (though Unusual)
While rare in simple reactions, it's theoretically possible to have multiple limiting reactants in complex reactions, particularly those involving multiple steps or simultaneous reactions. If the stoichiometric ratios between the reactants are such that two or more are consumed simultaneously, then all of them can be considered limiting reactants. This usually occurs in more intricate reaction pathways.
7. The Concept of Limiting Reactant Applies to Both Simple and Complex Reactions
Regardless of the complexity of the reaction – whether it's a simple synthesis or a multi-step, multi-component reaction – the concept of a limiting reactant always applies. Even in complex reactions with several intermediates and products, the reactant that gets consumed first and thereby limits the overall reaction yield is still considered the limiting reactant. However, identifying the limiting reactant in complex reactions might require more advanced techniques.
8. The Determination of the Limiting Reactant Often Involves Several Steps
Calculating the limiting reactant usually involves multiple steps:
- Balancing the chemical equation: Ensuring the equation reflects the correct stoichiometric ratios.
- Converting reactant masses or volumes to moles: Using molar masses and densities.
- Comparing the mole ratios of reactants to the stoichiometric ratios: This step determines which reactant's mole ratio is smallest relative to its stoichiometric coefficient. The reactant with the smallest ratio (after considering stoichiometric coefficients) is the limiting reactant.
- Calculating the theoretical yield: This is done using the moles of the limiting reactant and the stoichiometric ratios.
9. The Limiting Reactant is Independent of Reaction Rate
While the reaction rate might influence how quickly the limiting reactant is consumed, the identity of the limiting reactant itself is independent of the reaction rate. A fast reaction will consume the limiting reactant quickly, but a slow reaction will simply take longer to consume it; the amount consumed will still be determined by the initial amounts and stoichiometry.
10. Knowing the Limiting Reactant Helps Optimize Reaction Conditions
In industrial settings and laboratory experiments, identifying the limiting reactant is essential for optimizing reaction conditions and maximizing yield. By adjusting the quantities of reactants, we can ensure efficient use of resources and prevent waste. Knowing that a specific reactant is limiting allows chemists and engineers to focus on manipulating factors that affect the amount or reactivity of that reactant to increase the yield of the desired products.
Addressing Common Misconceptions
Several common misconceptions surround limiting reactants:
-
Misconception 1: The reactant with the smallest mass is always the limiting reactant. This is false. The limiting reactant is determined by the moles of each reactant and their stoichiometric ratios, not their masses. A reactant with a small mass but a high molar mass might be in excess, while a reactant with a large mass but a low molar mass might be limiting.
-
Misconception 2: The limiting reactant is always the reactant with the smallest number of moles. This is also false. The number of moles of each reactant needs to be compared to its stoichiometric coefficient in the balanced equation. A reactant might have fewer moles than another reactant but still be in excess if its stoichiometric coefficient is also smaller.
-
Misconception 3: The limiting reactant can be determined without a balanced chemical equation. This is incorrect. The balanced chemical equation provides the essential mole ratios, which are crucial for accurately identifying the limiting reactant.
Conclusion: Mastering Limiting Reactants
Understanding the statements that are always true about limiting reactants is paramount for mastering stoichiometry. By consistently applying these principles and avoiding common misconceptions, one gains the ability to accurately predict reaction yields, optimize experimental procedures, and develop a deeper understanding of chemical processes. Remember that the limiting reactant governs the maximum amount of product that can be formed, regardless of the quantities of other reactants present. This foundational concept underpins many crucial calculations and applications in chemistry and related fields.
Latest Posts
Latest Posts
-
A Bird Building Their Nest In A Tree
Mar 24, 2025
-
Different Conformations Of The Same Molecule
Mar 24, 2025
-
Does Gas Have A Fixed Shape
Mar 24, 2025
-
What Makes Each Amino Acid Unique
Mar 24, 2025
-
Is Oxygen A Metal Or Nonmetal Or Metalloid
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about What Statements Are Always True About Limiting Reactants . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
