How Many Lone Pairs Does H2o Have
Muz Play
Mar 22, 2025 · 5 min read
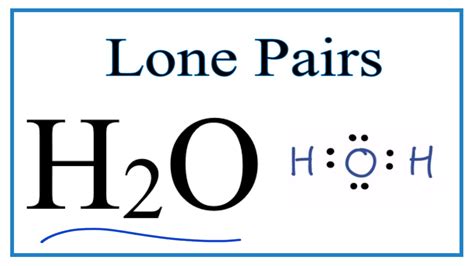
Table of Contents
How Many Lone Pairs Does H₂O Have? A Deep Dive into Water's Molecular Geometry
Water, the elixir of life, is a deceptively simple molecule with a surprisingly complex structure. Its seemingly straightforward chemical formula, H₂O, masks a fascinating interplay of electrons that dictates its unique properties and, consequently, its crucial role in our world. One of the key aspects of understanding water's behavior lies in determining the number of lone pairs of electrons on its central oxygen atom. This article will delve deep into the molecular geometry of water, explaining how to determine the number of lone pairs and exploring the implications of this structural feature.
Understanding Electron Domains and VSEPR Theory
Before we can count the lone pairs in water, we need to understand the fundamental principles governing molecular geometry. The Valence Shell Electron Pair Repulsion (VSEPR) theory is crucial here. This theory posits that electron pairs, both bonding and non-bonding (lone pairs), repel each other and arrange themselves around the central atom to minimize this repulsion, thus defining the molecule's shape.
Electron domains are regions of high electron density surrounding the central atom. These domains can be either bonding pairs (electrons shared between atoms in a covalent bond) or lone pairs (electrons not involved in bonding). The number of electron domains significantly influences the molecule's geometry.
Steps to Determine Electron Domains in H₂O:
-
Determine the Lewis Structure: The Lewis structure of water shows the oxygen atom (with six valence electrons) sharing one electron pair each with two hydrogen atoms (each with one valence electron). This leaves four electrons, or two lone pairs, on the oxygen atom.
-
Count the Electron Domains: In H₂O, the oxygen atom has two bonding pairs (one with each hydrogen atom) and two lone pairs. Therefore, there are a total of four electron domains around the oxygen atom.
-
Apply VSEPR Theory: Four electron domains predict a tetrahedral electron domain geometry. However, the molecular geometry (the arrangement of atoms only) is bent or V-shaped because the lone pairs take up more space than bonding pairs.
Visualizing the Lone Pairs in Water
Imagine the oxygen atom at the center of a tetrahedron. Two corners of the tetrahedron are occupied by hydrogen atoms, representing the bonding pairs. The remaining two corners are occupied by the lone pairs. These lone pairs, although not directly involved in bonding, exert a significant repulsive force, pushing the hydrogen atoms closer together and resulting in the characteristic bent shape.
The Impact of Lone Pairs on Bond Angle
The ideal bond angle in a tetrahedral structure is 109.5°. However, because of the greater repulsive force of the lone pairs compared to bonding pairs, the H-O-H bond angle in water is slightly less, approximately 104.5°. This compression is a direct consequence of the presence and repulsive effect of the lone pairs.
Why Understanding Lone Pairs is Crucial
The presence of two lone pairs on the oxygen atom in water is far more than a simple structural detail; it is the key to understanding many of water's remarkable properties:
-
High Boiling Point: The strong dipole moment created by the bent shape and the electronegativity difference between oxygen and hydrogen leads to strong hydrogen bonding between water molecules. The lone pairs on the oxygen atom are crucial for the formation of these hydrogen bonds, leading to water's unexpectedly high boiling point compared to other molecules of similar molecular weight.
-
High Surface Tension: Hydrogen bonding, facilitated by the lone pairs, creates a strong cohesive force between water molecules, resulting in a high surface tension. This allows insects to walk on water and explains capillary action in plants.
-
Excellent Solvent: Water's polarity, a direct consequence of its bent geometry and lone pairs, makes it an excellent solvent for many ionic and polar substances. The lone pairs on the oxygen can interact with positively charged ions or the positive ends of polar molecules, effectively dissolving them.
-
High Specific Heat Capacity: The hydrogen bonding network created by the lone pairs requires a significant amount of energy to break, resulting in water's high specific heat capacity. This property is essential for regulating temperature in living organisms and in large bodies of water.
-
Density Anomaly: The unique hydrogen bonding network in water, influenced by the lone pairs, leads to its anomalous behavior: ice is less dense than liquid water. This is critical for aquatic life, as ice floats, insulating the water below from extreme cold.
Beyond Water: Lone Pairs in Other Molecules
Understanding the impact of lone pairs extends beyond the simple case of water. Many molecules exhibit lone pairs on their central atoms, influencing their geometry and properties in similar ways. Ammonia (NH₃), for instance, has one lone pair on the nitrogen atom, leading to a trigonal pyramidal molecular geometry. Methane (CH₄), on the other hand, has no lone pairs, resulting in a tetrahedral geometry. The presence or absence of lone pairs and their influence on molecular geometry is a crucial concept in chemistry for predicting the properties of a wide range of compounds.
Conclusion: The Significance of Lone Pairs in H₂O
In conclusion, water (H₂O) possesses two lone pairs of electrons on its central oxygen atom. These lone pairs are not merely structural features; they are the fundamental cause of many of water's extraordinary properties. Understanding the role of lone pairs in determining molecular geometry and influencing intermolecular interactions is critical for comprehending the behavior of water and a vast array of other molecules. The VSEPR theory provides a powerful framework for predicting molecular geometry based on the number of electron domains, including both bonding and non-bonding pairs, highlighting the profound influence of these seemingly simple structural elements. From its high boiling point to its exceptional solvent properties, the two lone pairs on the oxygen atom in H₂O are central to the unique and life-sustaining characteristics of this remarkable molecule. This intricate dance of electrons underscores the elegant simplicity and profound complexity hidden within the seemingly simple formula, H₂O.
Latest Posts
Latest Posts
-
Where Are The Nonmetals Located On The Periodic Table
Mar 23, 2025
-
Does A Catalyst Lower Activation Energy
Mar 23, 2025
-
Is Boiling Point Physical Or Chemical Property
Mar 23, 2025
-
Graph Sine And Cosine Functions Worksheet
Mar 23, 2025
-
What Are The Two Types Of Forces In Classical Mechanics
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs Does H2o Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
