Does A Catalyst Lower Activation Energy
Muz Play
Mar 23, 2025 · 6 min read
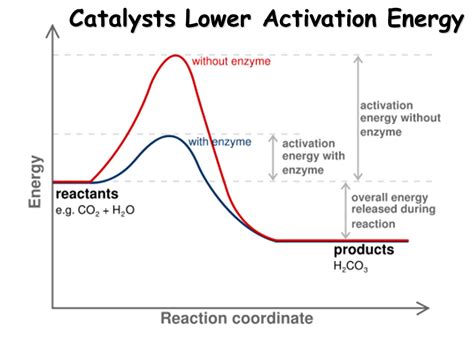
Table of Contents
Does a Catalyst Lower Activation Energy? A Deep Dive into Catalysis
Catalysis is a fundamental process in chemistry, impacting everything from industrial production to biological processes within our bodies. At the heart of catalysis lies the question: does a catalyst lower activation energy? The answer is a resounding yes, and understanding how it does so is crucial to appreciating the power and importance of catalysts. This article will delve into the intricacies of activation energy, the role of catalysts in lowering it, and the various mechanisms through which they achieve this remarkable feat. We'll explore different types of catalysts, their applications, and the broader implications of their ability to manipulate reaction rates.
Understanding Activation Energy: The Energy Barrier to Reaction
Chemical reactions don't spontaneously occur just because they're thermodynamically favorable. There's an energy hurdle that needs to be overcome – the activation energy (Ea). Think of it as the energy required to initiate a reaction, like pushing a boulder over a hill. The boulder represents the reactants, the hill's height represents the activation energy, and the valley on the other side represents the products.
Molecules need to possess a minimum amount of kinetic energy to successfully navigate this energy barrier and transform into products. Only molecules with energy exceeding the activation energy can successfully overcome this hurdle and participate in the reaction. This explains why many thermodynamically favorable reactions proceed incredibly slowly at room temperature – the activation energy is too high.
The Role of Transition States
The journey from reactants to products isn't a direct path. Molecules pass through a high-energy intermediate state called the transition state. The transition state is incredibly short-lived and unstable, representing the peak of the energy barrier. The activation energy is the difference in energy between the reactants and this transition state.
Catalysts: Lowering the Activation Energy
This is where catalysts come in. A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. It achieves this by providing an alternative reaction pathway with a lower activation energy. Instead of the boulder having to climb a steep hill, the catalyst creates a gentler slope, making it easier for the boulder (reactants) to reach the other side (products).
The key takeaway: A catalyst does not change the thermodynamics of a reaction; it doesn't alter the enthalpy change (ΔH) or the equilibrium constant (K). It solely affects the kinetics – the rate at which the reaction proceeds. It achieves this by lowering the activation energy, thereby increasing the fraction of molecules possessing sufficient energy to overcome the energy barrier.
Mechanisms of Catalyst Action: How Catalysts Lower Activation Energy
Catalysts employ several mechanisms to lower the activation energy. These mechanisms often involve the formation of intermediate complexes between the catalyst and the reactants, creating a more favorable pathway for the reaction to proceed.
1. Adsorption and Desorption: The Surface Effect
Many catalysts, particularly heterogeneous catalysts (catalysts in a different phase than the reactants), work by adsorbing reactant molecules onto their surface. This adsorption weakens bonds within the reactant molecules, making them more reactive. Once the reaction occurs on the catalyst surface, the products are desorbed, freeing the catalyst to participate in further reactions. This surface interaction significantly lowers the activation energy by providing a more accessible pathway for bond breaking and formation.
Think of it like bringing two shy people together in a comfortable setting (the catalyst's surface). The catalyst facilitates interaction, making it easier for them to bond (react).
2. Formation of Intermediate Complexes: Lowering the Energy Barrier
Homogeneous catalysts (catalysts in the same phase as the reactants) often form intermediate complexes with the reactants. These complexes have lower energy than the transition state of the uncatalyzed reaction. This effectively lowers the energy barrier, allowing the reaction to proceed more readily. The catalyst then breaks away from the product, ready to catalyze another reaction. This complex formation alters the reaction mechanism, providing a more energetically favorable route.
Imagine the catalyst as a matchmaker, bringing reactants together in a specific way that minimizes the energy required for them to react.
3. Orientation and Proximity: Facilitating Molecular Interactions
Catalysts can also enhance reaction rates by properly orienting reactant molecules, bringing them into closer proximity. In an uncatalyzed reaction, molecules must collide with the correct orientation and sufficient energy for the reaction to occur. Catalysts can hold reactant molecules in favorable orientations, increasing the probability of successful collisions and decreasing the activation energy needed.
This is akin to arranging dance partners in the ideal position for a waltz; the catalyst facilitates the interaction, ensuring success.
Types of Catalysts and Their Applications
Catalysts come in various forms, each designed to effectively lower the activation energy for specific types of reactions.
1. Homogeneous Catalysts: Blending In
Homogeneous catalysts exist in the same phase (e.g., liquid or gas) as the reactants. Examples include metal complexes used in many industrial processes, such as the production of polymers. Their advantage lies in their intimate interaction with reactants, leading to high catalytic activity. However, separating them from the products can be challenging.
2. Heterogeneous Catalysts: The Surface Advantage
Heterogeneous catalysts exist in a different phase from the reactants, usually a solid catalyst interacting with liquid or gaseous reactants. Examples include the platinum-based catalysts in catalytic converters, which convert harmful automobile exhaust gases into less harmful substances. Their ease of separation from products is a significant advantage.
3. Biocatalysts: Enzymes – Nature's Catalysts
Enzymes are biological catalysts, protein molecules that dramatically increase the rate of biochemical reactions within living organisms. Their remarkable specificity and efficiency are a testament to the power of catalysis in sustaining life. They lower activation energy through various mechanisms, including substrate binding and active site interactions.
Real-World Applications: The Impact of Catalysts
The ability of catalysts to lower activation energy has profound implications across various industries and scientific fields:
-
Chemical Industry: Catalysts are crucial for the efficient production of a vast array of chemicals, including fertilizers, plastics, and pharmaceuticals. They allow for faster reactions, lower operating temperatures, and reduced energy consumption, making industrial processes more economically viable and environmentally friendly.
-
Automotive Industry: Catalytic converters rely on heterogeneous catalysts to reduce harmful emissions from vehicles, contributing significantly to cleaner air.
-
Petroleum Refining: Catalysts are essential for refining crude oil into gasoline, diesel, and other valuable products. They facilitate complex reactions, maximizing yield and optimizing product quality.
-
Environmental Remediation: Catalysts are used to degrade pollutants in water and soil, helping to clean up contaminated environments.
-
Medicine: Enzymes are essential for numerous metabolic processes, and their catalytic activity is vital for life. Many drugs act by inhibiting or activating specific enzymes, impacting the rates of crucial biochemical reactions.
Conclusion: The Indispensable Role of Catalysts
The answer to "Does a catalyst lower activation energy?" is unequivocally yes. Catalysts are indispensable tools that significantly enhance reaction rates by providing alternative pathways with lower activation energies. Understanding how catalysts achieve this feat, through mechanisms like adsorption, complex formation, and orientational effects, is crucial for developing new catalysts and refining existing ones. Their impact extends far beyond the laboratory, permeating diverse sectors and playing a pivotal role in modern technology, industrial processes, and even the very processes of life itself. The continuous research and development in catalysis promise even more transformative applications in the future, shaping our world in profound ways.
Latest Posts
Latest Posts
-
Fes Compound Name With Roman Numerals
Mar 25, 2025
-
Metals Are Located Where On The Periodic Table
Mar 25, 2025
-
All Organisms Are Composed Of One Or More Cells
Mar 25, 2025
-
Anything That Interferes With Successful Communication Is Said To Be
Mar 25, 2025
-
Construct The Confidence Interval For The Population Mean M
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Does A Catalyst Lower Activation Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
