How Many Pi Electrons In A Double Bond
Muz Play
Mar 29, 2025 · 6 min read
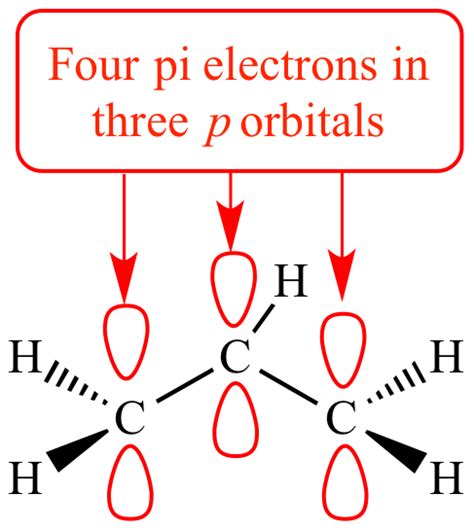
Table of Contents
How Many Pi Electrons in a Double Bond? Understanding Pi Bonds and Aromaticity
The question of how many pi electrons exist in a double bond is fundamental to understanding organic chemistry. It's a seemingly simple question with surprisingly nuanced answers, especially when considering the broader context of conjugated systems and aromaticity. This article delves deep into the concept of pi electrons in double bonds, explaining their importance in molecular structure, reactivity, and the properties of organic compounds.
Understanding Pi Bonds and Sigma Bonds
Before diving into the number of pi electrons, let's clarify the nature of double bonds themselves. A double bond consists of two types of bonds: a sigma (σ) bond and a pi (π) bond.
-
Sigma (σ) Bonds: These are strong, single bonds formed by the head-on overlap of atomic orbitals. They are the fundamental building blocks of all covalent bonds, including those within double and triple bonds. Sigma bonds allow free rotation around the bond axis.
-
Pi (π) Bonds: These are weaker bonds formed by the sideways overlap of p orbitals. They are found only in double and triple bonds. The sideways overlap creates regions of electron density above and below the plane of the sigma bond. Pi bonds restrict rotation around the bond axis, leading to the rigidity often observed in molecules with double bonds.
The Crucial Role of Pi Electrons
Pi electrons are the electrons involved in the pi bond. They are crucial because they dictate much of the molecule's reactivity and physical properties. Their delocalized nature, especially in conjugated systems, leads to unique characteristics. The number of pi electrons is therefore critical in determining the molecule's behavior.
How Many Pi Electrons in a Single Double Bond?
The answer is straightforward: a single double bond contains two pi electrons. These two electrons are the ones involved in the sideways overlap of the two p orbitals that form the pi bond. Remember, each bond has a pair of electrons. Therefore, a double bond with one sigma and one pi bond contains two electrons in the sigma bond and two electrons in the pi bond.
Beyond Single Double Bonds: Conjugated Systems
The situation becomes more complex when we consider molecules with multiple double bonds, especially when they are conjugated. Conjugated systems are molecules containing alternating single and double bonds, or those where a double bond is adjacent to a lone pair. In these systems, the pi electrons are not localized to a single double bond but are delocalized across the entire conjugated system. This delocalization significantly affects the molecule's properties.
Delocalization and Resonance
Delocalization of pi electrons is often represented using resonance structures. Resonance structures show different possible arrangements of the pi electrons, with the actual molecule being a hybrid of all these structures. The delocalized pi electrons are not assigned to specific bonds but are spread across the entire conjugated system.
For example, consider 1,3-butadiene (CH2=CH-CH=CH2). This molecule has two double bonds, each with its two pi electrons. However, due to conjugation, these four pi electrons are delocalized across all four carbon atoms, leading to increased stability.
Examples of Conjugated Systems with Delocalized Pi Electrons:
-
Benzene (C6H6): This classic example of aromaticity has a ring of six carbon atoms with alternating single and double bonds. It has six delocalized pi electrons distributed evenly throughout the ring. This delocalization is a key factor in benzene's exceptional stability.
-
1,3,5-Hexatriene: This molecule has three conjugated double bonds. Its six pi electrons are delocalized across the six carbon atoms.
Aromaticity: A Special Case of Delocalized Pi Electrons
Aromatic compounds are a special class of conjugated systems that exhibit exceptional stability due to a specific number of delocalized pi electrons. Hückel's rule provides a criterion for aromaticity: a planar, cyclic, conjugated system with (4n+2) pi electrons (where n is an integer) is aromatic.
Therefore, to be considered aromatic, a molecule must:
- Be cyclic.
- Be planar.
- Be conjugated.
- Possess (4n+2) pi electrons.
Benzene, with its six pi electrons (n=1), is a perfect example of an aromatic compound. Other examples include pyrrole, furan, and thiophene. Molecules that satisfy the first three criteria but have (4n) pi electrons are considered antiaromatic and are highly unstable.
Examples of Aromatic Compounds and Their Pi Electron Count:
- Benzene (C6H6): 6 pi electrons (n=1)
- Pyrrole (C4H4NH): 6 pi electrons (n=1; including the lone pair on nitrogen)
- Furan (C4H4O): 6 pi electrons (n=1; including the lone pair on oxygen)
- Thiophene (C4H4S): 6 pi electrons (n=1; including the lone pair on sulfur)
- Naphthalene (C10H8): 10 pi electrons (n=2)
Counting Pi Electrons in More Complex Molecules
Counting pi electrons in more complex molecules requires careful consideration of the molecular structure and the presence of conjugated systems. Here's a systematic approach:
-
Identify all double and triple bonds: Each double bond contributes two pi electrons, and each triple bond contributes four pi electrons.
-
Identify lone pairs involved in conjugation: Lone pairs on atoms adjacent to a double bond can participate in conjugation, contributing their electrons to the pi system.
-
Draw resonance structures (if applicable): Resonance structures help to visualize the delocalization of pi electrons in conjugated systems.
-
Determine the total number of pi electrons: Add up the contributions from all double bonds, triple bonds, and conjugated lone pairs.
-
Assess aromaticity (if applicable): Check if the molecule meets Hückel's rule for aromaticity.
Practical Applications of Understanding Pi Electrons
The understanding of pi electrons and their delocalization has significant practical applications in various fields:
-
Drug design: Many pharmaceuticals contain conjugated systems with delocalized pi electrons. Understanding these systems is essential in designing and modifying drugs for enhanced efficacy and reduced side effects.
-
Materials science: Conjugated polymers, with their extended pi systems, exhibit unique electrical and optical properties. This makes them essential for applications like organic light-emitting diodes (OLEDs) and solar cells.
-
Spectroscopy: The presence and delocalization of pi electrons strongly influence a molecule's UV-Vis absorption spectrum. Analyzing these spectra helps in identifying and characterizing organic compounds.
Conclusion
The seemingly simple question of how many pi electrons are in a double bond opens a doorway to a deeper understanding of molecular structure, reactivity, and the fascinating world of organic chemistry. While a single double bond has two pi electrons, the complexity increases significantly in conjugated systems, where delocalization plays a crucial role. A firm grasp of pi electrons is vital for anyone studying or working in fields involving organic molecules, from drug discovery to material science. This understanding provides the foundation for comprehending the diverse properties and behaviors of countless organic compounds. By mastering the concepts of sigma and pi bonds, resonance, and aromaticity, you'll be well-equipped to tackle more challenging concepts in organic chemistry and its related fields.
Latest Posts
Latest Posts
-
Ionic Compounds Dissociate In Water Into
Mar 31, 2025
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
-
Is Ph A Chemical Or Physical Property
Mar 31, 2025
-
Identify The Characteristics Of The Hydroboration Oxidation Of An Alkene
Mar 31, 2025
-
What Are The Differences Between The Pulmonary And Systemic Circulation
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Pi Electrons In A Double Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
