How Many Protons Are In Sulfur
Muz Play
Mar 18, 2025 · 5 min read
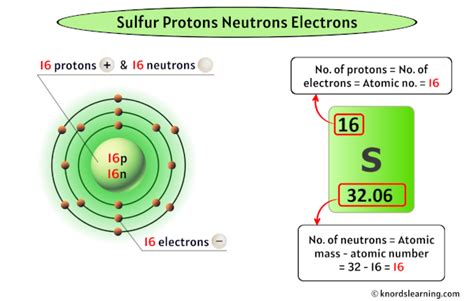
Table of Contents
How Many Protons Are in Sulfur? Exploring the Atomic Structure of Sulfur
Determining the number of protons in an atom is fundamental to understanding its identity and properties. This article delves deep into the question: How many protons are in sulfur? We'll explore the concept of atomic number, delve into the structure of the sulfur atom, and examine its significance in chemistry and beyond. We'll also touch on related concepts to provide a comprehensive understanding of sulfur's place in the periodic table and the wider world of atomic structure.
Understanding Atomic Number and Protons
The key to answering "How many protons are in sulfur?" lies in understanding the concept of atomic number. The atomic number of an element is the number of protons found in the nucleus of each atom of that element. Protons, along with neutrons, reside in the atom's nucleus, while electrons orbit around it. Protons carry a positive charge, electrons carry a negative charge, and neutrons are neutral.
The atomic number is unique to each element and defines its identity. It's the fundamental building block that differentiates one element from another. If you change the number of protons, you fundamentally change the element itself. Adding a proton transforms sulfur into another element entirely.
Sulfur's Atomic Number and Proton Count
The atomic number of sulfur is 16. This means that every sulfur atom contains 16 protons in its nucleus. This is a fundamental and unchanging characteristic of sulfur. No matter where you find sulfur – in a volcano, in your body, or in a laboratory – each atom will always have 16 protons.
Isotopes and Neutrons: A Closer Look
While the number of protons defines the element, the number of neutrons can vary. Atoms of the same element with differing numbers of neutrons are called isotopes. Sulfur has several isotopes, including sulfur-32 (the most abundant), sulfur-33, sulfur-34, and sulfur-36. These isotopes all have 16 protons but differ in their neutron count.
The total number of protons and neutrons in an atom's nucleus is called its mass number. For example, sulfur-32 has a mass number of 32 (16 protons + 16 neutrons), while sulfur-34 has a mass number of 34 (16 protons + 18 neutrons). While isotopes have different mass numbers, they remain the same element because they possess the defining characteristic: 16 protons.
The Importance of Protons in Determining Sulfur's Properties
The number of protons in a sulfur atom significantly influences its chemical and physical properties. This is because the protons determine the number of electrons in a neutral atom. Electrons are responsible for chemical bonding and reactivity. Sulfur's 16 protons mean it has 16 electrons, arranged in specific energy levels or shells. This electron configuration dictates how sulfur interacts with other atoms.
Sulfur's Chemical Behavior: A Consequence of Proton Number
The arrangement of sulfur's 16 electrons leads to its characteristic chemical behavior. It readily forms covalent bonds with other atoms, often sharing electrons to achieve a stable electron configuration. This explains why sulfur is found in many different compounds, from sulfur dioxide (SO₂) in air pollution to the sulfur-containing amino acids that are essential building blocks of proteins in living organisms. This ability to form bonds is directly linked to its atomic structure, determined by the 16 protons.
Sulfur's Physical Properties: Indirect Influence of Protons
While the number of protons directly determines the chemical behavior, it indirectly influences physical properties. The number of electrons affects the strength of intermolecular forces, influencing physical properties like melting point and boiling point. Sulfur's physical properties, such as its yellow color and its tendency to exist in various allotropic forms (different structural arrangements), are a consequence of its electron configuration, which is determined by its 16 protons.
Sulfur in the Periodic Table: Its Position and Significance
Sulfur's position in the periodic table further underscores the significance of its 16 protons. It resides in Group 16 (also known as the chalcogens) and Period 3. Its position reflects its chemical properties: it's a nonmetal that readily forms anions (negatively charged ions) by gaining electrons to achieve a stable octet (eight electrons in its outermost shell). The periodic trend of electronegativity (an atom's ability to attract electrons) also increases as you move across a period; sulfur's position highlights its moderate electronegativity compared to other elements in its period.
Applications of Sulfur and its Compounds
The unique properties of sulfur, arising from its 16 protons, make it vital for numerous applications across various industries:
1. Industrial Applications:
- Vulcanization of Rubber: Sulfur is crucial in vulcanizing rubber, giving it strength and elasticity. This process involves cross-linking the polymer chains of rubber using sulfur atoms.
- Sulphuric Acid Production: Sulphuric acid (H₂SO₄), one of the most important industrial chemicals, is produced using sulfur as a starting material. Its widespread use in fertilizer production, metal refining, and countless other industrial processes underlines the significance of sulfur.
- Production of Other Chemicals: Sulfur is a precursor to many other important chemicals, including various pesticides, detergents, and dyes.
2. Biological Significance:
- Amino Acids: Sulfur is a component of several essential amino acids, such as cysteine and methionine, which are fundamental building blocks of proteins. These amino acids play crucial roles in protein structure and function within living organisms.
- Enzymes: Sulfur is also a part of certain enzymes, which are biological catalysts essential for various metabolic processes.
- Other Biological Roles: Sulfur plays various other roles in biological systems, including the formation of disulfide bonds, which contribute to the structural stability of proteins.
Conclusion: The Defining Role of 16 Protons
The number of protons in an atom is paramount in defining its properties and behavior. For sulfur, the presence of 16 protons dictates its atomic number, its chemical reactivity, its physical properties, and its wide-ranging applications in industry and biology. Understanding the significance of this number provides a deeper appreciation of the role of sulfur in the world around us. From the rubber in our tires to the proteins in our bodies, the 16 protons in each sulfur atom play a fundamental role in shaping the materials and processes that underpin our lives. Therefore, the answer to "How many protons are in sulfur?" is not just a simple number; it's a key to understanding a vital element. This number dictates its place in the periodic table, its chemical properties, and its essential role in diverse aspects of our world.
Latest Posts
Latest Posts
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
-
What Are The Five Evaluation Criteria
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Are In Sulfur . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
