How Many Radial Nodes Are Present In This Orbital
Muz Play
Mar 27, 2025 · 5 min read
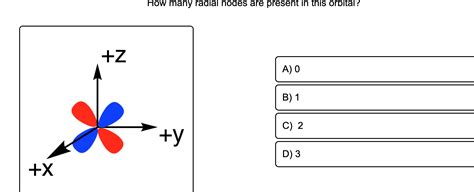
Table of Contents
How Many Radial Nodes Are Present in This Orbital? A Deep Dive into Atomic Orbitals and Quantum Numbers
Determining the number of radial nodes in an atomic orbital is a fundamental concept in chemistry, crucial for understanding electron behavior and the shapes of orbitals. This article will provide a comprehensive guide to understanding radial nodes, their relationship to quantum numbers, and how to calculate them for any given orbital.
Understanding Atomic Orbitals and Quantum Numbers
Before diving into radial nodes, let's establish a solid foundation. Electrons in atoms don't move in predictable paths like planets around the sun. Instead, they occupy regions of space called atomic orbitals, which are described mathematically by wave functions. These wave functions are characterized by four quantum numbers:
-
Principal Quantum Number (n): This determines the energy level and size of the orbital.
ncan be any positive integer (1, 2, 3...). Highernvalues mean higher energy and larger orbitals. -
Azimuthal Quantum Number (l): This determines the shape of the orbital and the orbital angular momentum.
lcan range from 0 ton-1.l = 0corresponds to an s orbital (spherical),l = 1to a p orbital (dumbbell-shaped),l = 2to a d orbital (more complex shapes), and so on. -
Magnetic Quantum Number (ml): This determines the orientation of the orbital in space.
mlcan range from -lto +l, including 0. For example, a p orbital (l = 1) has three possible orientations (ml = -1, 0, +1), corresponding to the px, py, and pz orbitals. -
Spin Quantum Number (ms): This describes the intrinsic angular momentum of the electron, often referred to as its "spin". It can have a value of +1/2 or -1/2.
Radial Nodes and Angular Nodes: Defining the Orbital's Structure
The wave function of an atomic orbital can be expressed as a product of two functions: a radial function and an angular function.
-
Radial function: This function describes the probability of finding an electron at a particular distance from the nucleus. Radial nodes are points where the radial wave function equals zero. These are spherical surfaces where the probability of finding the electron is zero.
-
Angular function: This function describes the shape of the orbital. Angular nodes are planes where the angular wave function equals zero. The number of angular nodes is equal to the azimuthal quantum number,
l.
Calculating the Number of Radial Nodes
The number of radial nodes in an orbital is determined by the principal quantum number (n) and the azimuthal quantum number (l):
Number of radial nodes = n - l - 1
Let's break this down:
- n (Principal Quantum Number): Represents the total number of nodes (both radial and angular).
- l (Azimuthal Quantum Number): Represents the number of angular nodes.
- n - l - 1: This subtraction gives us the remaining nodes, which are the radial nodes.
Examples:
Let's apply this formula to some common orbitals:
-
1s orbital (n = 1, l = 0): Number of radial nodes = 1 - 0 - 1 = 0. The 1s orbital has no radial nodes.
-
2s orbital (n = 2, l = 0): Number of radial nodes = 2 - 0 - 1 = 1. The 2s orbital has one radial node.
-
2p orbital (n = 2, l = 1): Number of radial nodes = 2 - 1 - 1 = 0. The 2p orbital has zero radial nodes.
-
3s orbital (n = 3, l = 0): Number of radial nodes = 3 - 0 - 1 = 2. The 3s orbital has two radial nodes.
-
3p orbital (n = 3, l = 1): Number of radial nodes = 3 - 1 - 1 = 1. The 3p orbital has one radial node.
-
3d orbital (n = 3, l = 2): Number of radial nodes = 3 - 2 - 1 = 0. The 3d orbital has zero radial nodes.
Visualizing Radial Nodes
Imagine the radial wave function as a graph plotting electron probability density against distance from the nucleus. Radial nodes appear as points where the graph crosses the x-axis (probability density = 0). Between these nodes, the probability density is positive or negative, indicating regions of high and low electron probability. For s orbitals, these regions alternate between high probability near the nucleus and further out.
Significance of Radial Nodes
The presence and location of radial nodes significantly influence the properties of atomic orbitals:
-
Electron Density: Radial nodes divide the orbital into regions with different electron densities. The probability of finding an electron is higher in regions between nodes and zero at the nodes themselves.
-
Penetration: Electrons in orbitals with more radial nodes have a greater chance of penetrating closer to the nucleus. This affects shielding and effective nuclear charge experienced by outer electrons.
-
Energy Levels: Orbitals with more nodes generally have higher energy levels.
-
Chemical Reactivity: The distribution of electron density, as influenced by radial nodes, plays a crucial role in chemical bonding and reactivity.
Advanced Concepts and Considerations:
The concept of radial nodes extends to more complex systems beyond simple hydrogen-like atoms. In multi-electron atoms, electron-electron repulsions complicate the precise determination of radial node locations. However, the fundamental principles outlined above remain relevant. Computational methods are often employed to accurately model and visualize electron density in multi-electron systems.
Conclusion:
Understanding radial nodes is essential for a complete comprehension of atomic orbitals and their properties. By using the simple formula n - l - 1, we can quickly determine the number of radial nodes for any given orbital. This knowledge helps us visualize electron distribution, predict chemical behavior, and appreciate the intricacies of quantum mechanics in shaping the world around us. Remember, the location and number of radial nodes are critical in defining the unique characteristics of each atomic orbital and its contribution to the overall electronic structure of an atom. This foundational understanding empowers deeper explorations of chemical bonding, molecular geometry, and spectroscopic properties.
Latest Posts
Latest Posts
-
Where Does Dna Replication Take Place In A Eukaryotic Cell
Mar 30, 2025
-
Find Standard Matrix Of Linear Transformation
Mar 30, 2025
-
Is Delta H Products Minus Reactants
Mar 30, 2025
-
What Color Does Iodine Turn In The Presence Of Starch
Mar 30, 2025
-
How To Draw Lewis Structures For Ionic Compounds
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Radial Nodes Are Present In This Orbital . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
