How Many Sigma Bonds In A Triple Bond
Muz Play
Mar 18, 2025 · 6 min read
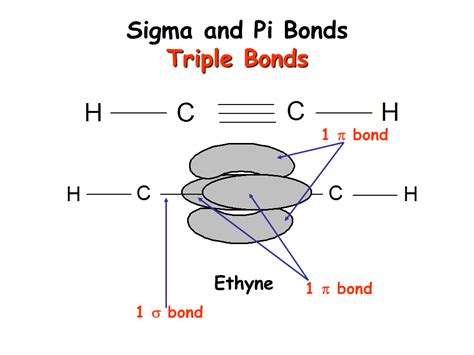
Table of Contents
How Many Sigma Bonds in a Triple Bond? Understanding Chemical Bonding
The question of how many sigma bonds exist within a triple bond is fundamental to understanding chemical bonding and molecular structure. It's a seemingly simple question, yet it unveils the intricacies of covalent bonding and its impact on molecular properties. This article delves deep into the concept of sigma (σ) and pi (π) bonds, exploring their formation, characteristics, and the specific case of triple bonds. We'll unravel the mysteries behind this crucial aspect of chemistry, providing a comprehensive understanding suitable for students and enthusiasts alike.
Understanding Sigma (σ) and Pi (π) Bonds: The Building Blocks of Covalent Bonds
Covalent bonds are formed when two atoms share one or more pairs of electrons. These bonds are not all created equal; they exhibit distinct types based on the orbital overlap involved. The two primary types are sigma (σ) and pi (π) bonds.
Sigma (σ) Bonds: The Foundation of Covalent Bonds
A sigma bond is formed by the head-on overlap of atomic orbitals. This means the electron density is concentrated along the internuclear axis – the imaginary line connecting the two atomic nuclei. Sigma bonds are the strongest type of covalent bond because of this direct and substantial overlap. They are crucial for the structural integrity of molecules. Regardless of the type of orbital involved (s, p, hybrid), the head-on overlap always defines a sigma bond.
Pi (π) Bonds: Adding Strength and Complexity
A pi (π) bond is formed by the sideways overlap of atomic p orbitals. Unlike sigma bonds, the electron density in a pi bond is concentrated above and below the internuclear axis. Pi bonds are generally weaker than sigma bonds due to the less effective orbital overlap. Importantly, pi bonds cannot exist independently; they always accompany a sigma bond. This is because the sideways overlap requires the atoms to already be bonded by a sigma bond, providing the necessary proximity for the p orbitals to interact.
Delving into Triple Bonds: A Symphony of Sigma and Pi Bonds
A triple bond is a type of covalent bond where three pairs of electrons are shared between two atoms. This strong bond significantly influences the properties of the molecules that contain it. Crucially, a triple bond always consists of one sigma bond and two pi bonds.
The Composition of a Triple Bond: One Sigma, Two Pi
Let's visualize this using the quintessential example: the nitrogen molecule (N₂). Each nitrogen atom possesses three unpaired electrons in its valence shell (2p orbitals). The formation of a triple bond proceeds as follows:
-
Sigma Bond Formation: One pair of electrons from each nitrogen atom overlaps head-on, forming a strong sigma bond. This sigma bond is the foundation upon which the triple bond is built. This direct overlap provides the most significant electron density along the bond axis.
-
Pi Bond Formation: The remaining two pairs of electrons from each nitrogen atom participate in sideways overlap, forming two pi bonds. These pi bonds are weaker than the sigma bond but collectively contribute significantly to the overall bond strength and stability. The electron density in these pi bonds is situated above and below the sigma bond, effectively surrounding the internuclear axis.
This arrangement—one sigma bond and two pi bonds—results in the characteristic short and strong triple bond observed in N₂ and other molecules with triple bonds.
Illustrative Examples: Triple Bonds in Action
Numerous molecules exhibit triple bonds, highlighting their significance in organic and inorganic chemistry. Let's explore a few prominent examples:
-
Nitrogen Gas (N₂): As previously discussed, the nitrogen molecule showcases a classic triple bond, exhibiting remarkable stability due to the strength of this bond. This high stability explains nitrogen's inertness in many chemical reactions.
-
Acetylene (Ethyne, C₂H₂): This simplest alkyne contains a carbon-carbon triple bond. The sp hybridization of the carbon atoms facilitates the formation of this triple bond, resulting in a linear molecular geometry.
-
Cyanide Ion (CN⁻): This ion possesses a carbon-nitrogen triple bond. The strong bond contributes to the toxicity and reactivity of this ion.
-
Nitriles (R-C≡N): A vast class of organic compounds containing a carbon-nitrogen triple bond. These compounds are widely utilized in various applications.
-
Carbon Monoxide (CO): While not strictly a triple bond in the same sense as N₂, CO exhibits a bond order of three, representing a combination of one sigma and two pi bonds. This strength underlies the toxicity of carbon monoxide.
In each of these cases, the underlying principle remains consistent: one sigma bond and two pi bonds comprise a triple bond. The specific properties of the molecule will vary depending on the atoms involved and their associated electron configurations, but the fundamental bond structure always remains the same.
Why is Understanding Sigma and Pi Bonds Important?
The ability to differentiate between and understand sigma and pi bonds is crucial for several reasons:
-
Predicting Molecular Geometry: The presence and arrangement of sigma and pi bonds significantly impact a molecule's three-dimensional structure. Sigma bonds predominantly define the skeletal arrangement, while pi bonds influence the overall shape and reactivity.
-
Explaining Molecular Properties: The strength and characteristics of sigma and pi bonds directly impact the physical and chemical properties of molecules, including boiling point, melting point, reactivity, and spectral behavior. Stronger bonds often translate to higher melting and boiling points, increased stability, and lower reactivity.
-
Understanding Chemical Reactions: The breaking and formation of sigma and pi bonds are central to virtually all chemical reactions. Understanding this process is crucial for predicting reaction mechanisms and outcomes.
-
Advanced Chemical Concepts: Concepts like resonance, aromaticity, and conjugation are fundamentally linked to the understanding of pi bonds and their interaction with sigma bonds.
Addressing Potential Misconceptions
Sometimes, confusion arises regarding the number of bonds in a triple bond. It's essential to clarify a few potential misconceptions:
-
Counting Electron Pairs vs. Bonds: A triple bond involves three pairs of electrons, but it's important to remember that it's composed of one sigma and two pi bonds, not three sigma bonds. Each bond represents a distinct type of orbital overlap.
-
The Role of Hybrid Orbitals: The hybridization of atomic orbitals (like sp, sp², sp³) affects the geometry and type of bonds formed. However, the fundamental categorization of bonds into sigma and pi remains the same regardless of hybridization.
Conclusion: The Significance of Triple Bonds and Their Components
The presence of a triple bond significantly impacts a molecule’s properties and reactivity. Understanding that a triple bond always consists of one sigma and two pi bonds is paramount for comprehending molecular structure and function. This fundamental knowledge lays the groundwork for tackling more complex chemical concepts and applications. Whether you're studying organic chemistry, inorganic chemistry, or biochemistry, the concept of sigma and pi bonds, and their role in triple bonds, is an indispensable element for success. By grasping this fundamental concept, you open the door to a deeper understanding of the fascinating world of chemical bonding and molecular interactions. Continued exploration of these concepts will lead to a richer appreciation of the intricate and beautiful world of chemistry.
Latest Posts
Latest Posts
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
-
Is Salt Water A Pure Substance
Mar 18, 2025
-
What Are The Functions Of The Family
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Sigma Bonds In A Triple Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
