How Many Valence Electrons Are In Sulfur
Muz Play
Mar 18, 2025 · 6 min read
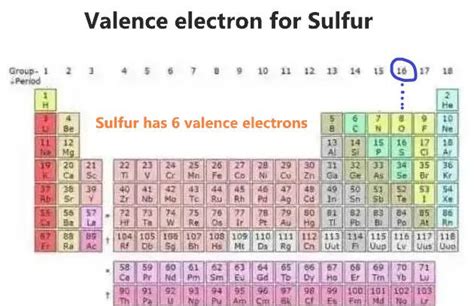
Table of Contents
How Many Valence Electrons Are in Sulfur? A Deep Dive into Atomic Structure and Chemical Bonding
Sulfur, a vibrant yellow nonmetal abundant in nature, plays a crucial role in various biological and industrial processes. Understanding its chemical behavior hinges on comprehending its electronic structure, particularly the number of valence electrons it possesses. This article will delve deep into the question: How many valence electrons are in sulfur? We'll explore the underlying principles of atomic structure, the significance of valence electrons in chemical bonding, and sulfur's unique characteristics stemming from its electron configuration.
Understanding Atomic Structure: The Foundation of Valence Electrons
Before tackling sulfur specifically, let's establish a fundamental understanding of atomic structure. Atoms are composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons occupy specific energy levels or shells, with each shell capable of holding a limited number of electrons. The outermost shell, known as the valence shell, houses the valence electrons. These electrons are the key players in chemical reactions, determining an element's reactivity and the types of bonds it can form.
The number of protons in an atom's nucleus defines its atomic number and dictates the element's identity. For a neutral atom, the number of electrons equals the number of protons. Electrons fill the energy levels starting with the lowest energy level and progressing outwards. The specific arrangement of electrons in an atom is called its electron configuration.
Electron Shells and Subshells
The electron shells are further divided into subshells, designated as s, p, d, and f. Each subshell can accommodate a specific number of electrons:
- s subshell: holds a maximum of 2 electrons
- p subshell: holds a maximum of 6 electrons
- d subshell: holds a maximum of 10 electrons
- f subshell: holds a maximum of 14 electrons
The filling of these subshells follows specific rules, including the Aufbau principle (filling orbitals in order of increasing energy), Hund's rule (maximizing unpaired electrons in a subshell), and the Pauli exclusion principle (no two electrons can have the same set of quantum numbers).
Determining Sulfur's Valence Electrons
Sulfur (S) has an atomic number of 16, meaning a neutral sulfur atom possesses 16 electrons. To determine the number of valence electrons, we need to understand its electron configuration. Following the rules of electron filling, sulfur's electron configuration is: 1s²2s²2p⁶3s²3p⁴.
Let's break this down:
- 1s²: Two electrons in the first energy level (s subshell).
- 2s²: Two electrons in the second energy level (s subshell).
- 2p⁶: Six electrons in the second energy level (p subshell).
- 3s²: Two electrons in the third energy level (s subshell).
- 3p⁴: Four electrons in the third energy level (p subshell).
The valence electrons are those in the outermost energy level, which in sulfur's case is the third energy level (n=3). This level contains the 3s² and 3p⁴ electrons. Adding these together (2 + 4 = 6), we find that sulfur has 6 valence electrons.
The Significance of Sulfur's Six Valence Electrons
The six valence electrons are the key to understanding sulfur's chemical behavior. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas with a full outer shell (octet rule). Sulfur, needing two more electrons to achieve a stable octet, readily forms chemical bonds to gain these electrons.
Chemical Bonding and Sulfur
Sulfur's six valence electrons allow it to participate in various types of chemical bonding, including:
-
Covalent Bonding: Sulfur commonly forms covalent bonds by sharing electrons with other atoms. This is because sharing electrons allows both atoms to achieve a more stable electron configuration. Examples include the formation of sulfur dioxide (SO₂) and hydrogen sulfide (H₂S). In these molecules, sulfur shares electrons with oxygen and hydrogen atoms, respectively.
-
Ionic Bonding: While less common than covalent bonding, sulfur can participate in ionic bonding under certain circumstances. In this type of bonding, sulfur can gain two electrons to form a sulfide ion (S²⁻), achieving a stable octet. This usually occurs when reacting with highly electropositive metals like sodium (Na) or potassium (K), leading to the formation of ionic compounds such as sodium sulfide (Na₂S).
-
Coordinate Covalent Bonding: Sulfur can also participate in coordinate covalent bonding, where both electrons shared in the bond originate from the same atom (sulfur in this case). This type of bonding is frequently observed in sulfur-containing compounds.
Sulfur's Diverse Chemical Properties
The ability of sulfur to form various types of chemical bonds and its six valence electrons contribute to its wide range of chemical properties and its diverse roles in different chemical compounds and biological systems. Sulfur's presence in various organic and inorganic molecules significantly impacts their properties and reactivity.
Sulfur in Nature and its Applications
Sulfur is found extensively in nature, both in its elemental form and as part of various compounds. It's a crucial component of many minerals, including sulfides and sulfates. Elementally, sulfur exists as S₈ molecules (eight sulfur atoms forming a ring).
The applications of sulfur and sulfur-containing compounds are vast and varied, spanning across numerous industries:
-
Industrial Uses: Sulfur is a vital raw material in the production of sulfuric acid (H₂SO₄), one of the most important industrial chemicals. Sulfuric acid is used extensively in fertilizer production, petroleum refining, and metal processing. Sulfur is also used in the vulcanization of rubber, enhancing its durability and elasticity.
-
Biological Roles: Sulfur is an essential element for all living organisms. It's a crucial component of several amino acids (e.g., cysteine and methionine), proteins, and enzymes. These sulfur-containing molecules play vital roles in various biological processes, including protein structure, enzyme activity, and metabolic pathways.
-
Medical Applications: Sulfur-containing compounds have been used in various medical applications, including topical treatments for skin conditions like acne and psoriasis. Some sulfur-based drugs are also employed to treat certain infections.
Conclusion: The Importance of Valence Electrons in Chemistry
Understanding the number of valence electrons in an atom, in this case, sulfur's six valence electrons, is crucial for comprehending its chemical behavior and its role in forming various compounds. The implications extend far beyond the realm of theoretical chemistry. Knowing the number of valence electrons allows chemists to predict the type and number of bonds an atom will form, its reactivity, and ultimately its chemical properties. Sulfur's six valence electrons directly influence its diverse applications in various industries and its essential role in biological systems, highlighting the fundamental significance of understanding atomic structure and electron configurations in chemistry. This detailed exploration underscores the interconnectedness of atomic structure, chemical bonding, and the practical applications of chemical knowledge. The seemingly simple question, "How many valence electrons are in sulfur?", opens a door to a vast and fascinating world of chemistry and its impact on our daily lives.
Latest Posts
Latest Posts
-
How To Calculate Percent Of Water In A Hydrate
Mar 19, 2025
-
Shear Force Diagram Of Cantilever Beam
Mar 19, 2025
-
Mean And Variance Of Sample Mean
Mar 19, 2025
-
Predict The Product For The Reaction Shown
Mar 19, 2025
-
When Is Work Positive And Negative
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Are In Sulfur . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
