How Many Valence Electrons Does He Have
Muz Play
Mar 18, 2025 · 6 min read
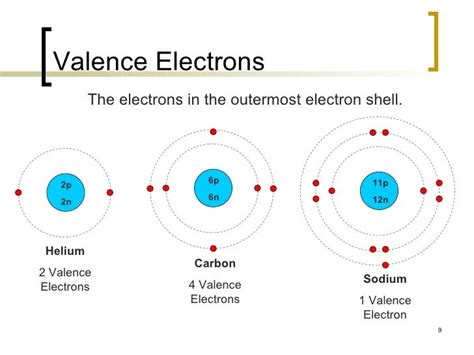
Table of Contents
How Many Valence Electrons Does He Have? Understanding Valence Electrons and Their Importance
The question, "How many valence electrons does he have?" implies a deeper inquiry into the fundamental nature of atoms and their chemical behavior. To answer this, we need to understand what valence electrons are and how they determine an element's properties. This article will delve into the concept of valence electrons, explaining their significance in chemical bonding, reactivity, and the periodic table's organization. We'll also explore how to determine the number of valence electrons for various elements, including the element "He" (helium), which is frequently used as an example.
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (also known as the valence shell) of an atom. These electrons are crucial because they participate directly in chemical bonding, determining an atom's reactivity and the types of bonds it can form. They are the electrons furthest from the nucleus and therefore experience the least attraction to the positively charged protons within the nucleus. This makes them more readily available to interact with other atoms.
Think of valence electrons as the "social butterflies" of the atom. They're the ones that interact with other atoms, forming connections and relationships (chemical bonds). The inner electrons, on the other hand, are more like the atom's "homebodies," staying close to the nucleus and remaining relatively uninvolved in external interactions.
The Importance of Valence Electrons in Chemical Bonding
The number of valence electrons an atom possesses directly influences the types of chemical bonds it can form. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas (Group 18 elements). This stable configuration is typically achieved by having eight valence electrons (the octet rule), although there are exceptions, particularly with elements in the first and second periods of the periodic table.
Atoms achieve this stability through various bonding mechanisms:
-
Ionic Bonding: This involves the transfer of electrons from one atom to another. Atoms with few valence electrons (e.g., alkali metals) tend to lose electrons, becoming positively charged ions (cations), while atoms with nearly full valence shells (e.g., halogens) tend to gain electrons, becoming negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions forms an ionic bond.
-
Covalent Bonding: This involves the sharing of electrons between atoms. Atoms with a moderate number of valence electrons often share electrons to achieve a stable octet. This sharing creates a covalent bond, forming molecules.
-
Metallic Bonding: This type of bonding occurs in metals, where valence electrons are delocalized and form a "sea" of electrons surrounding positively charged metal ions. This allows for the characteristic properties of metals like conductivity and malleability.
Determining the Number of Valence Electrons
There are several ways to determine the number of valence electrons an atom has:
-
Using the Periodic Table: The most straightforward method is by using the periodic table's group number (vertical columns). For main group elements (Groups 1-18, excluding transition metals), the group number generally corresponds to the number of valence electrons. For instance, Group 1 elements (alkali metals) have one valence electron, Group 2 elements (alkaline earth metals) have two, and so on. Group 18 elements (noble gases) have eight valence electrons (except helium, which has two). This is a simplified rule and does not apply to transition metals or inner transition metals.
-
Using Electron Configuration: Electron configuration shows how electrons are distributed among different energy levels and subshells within an atom. The outermost shell's electrons represent the valence electrons. For example, the electron configuration of oxygen is 1s²2s²2p⁴. The outermost shell is the second shell (n=2), containing 6 electrons (2s²2p⁴), therefore oxygen has 6 valence electrons.
-
Using Lewis Dot Structures: Lewis dot structures provide a visual representation of valence electrons. The element symbol represents the nucleus and inner electrons, while dots surrounding the symbol represent valence electrons. For example, oxygen's Lewis dot structure would have six dots surrounding the "O" symbol.
How Many Valence Electrons Does Helium (He) Have?
Helium (He), a noble gas, is a crucial element for understanding valence electrons and exceptions to the octet rule. Helium's atomic number is 2, meaning it has two protons and two electrons. Its electron configuration is 1s². This means both of its electrons occupy the first electron shell (n=1), which is also its outermost shell. Therefore, helium has two valence electrons. This explains its inertness and stability – its outermost shell is completely filled, satisfying the duet rule for the first electron shell. The duet rule applies to elements in the first period (hydrogen and helium), where a stable electron configuration is achieved with two electrons in the first shell.
Valence Electrons and Periodic Trends
The number of valence electrons significantly influences various periodic trends:
-
Atomic Radius: Atomic radius generally decreases across a period (left to right) and increases down a group (top to bottom). This trend is partially due to the increasing nuclear charge and the number of energy levels occupied by electrons. Elements with more valence electrons experience a stronger attraction to the nucleus, resulting in a smaller atomic radius.
-
Ionization Energy: Ionization energy is the energy required to remove an electron from an atom. It generally increases across a period and decreases down a group. Elements with more valence electrons tend to have higher ionization energies because removing an electron requires overcoming the stronger attractive force from the nucleus.
-
Electronegativity: Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. It generally increases across a period and decreases down a group. Elements with nearly full valence shells (like halogens) have high electronegativity, while elements with few valence electrons (like alkali metals) have low electronegativity.
Conclusion: The Significance of Valence Electrons
Understanding valence electrons is fundamental to grasping chemical reactivity and bonding. The number of valence electrons determines how an atom will interact with other atoms, leading to the formation of diverse compounds and materials with unique properties. The periodic table provides a valuable tool for predicting the number of valence electrons, and other concepts, like electron configuration and Lewis dot structures, provide more detailed insights into atomic structure and behavior. Helium's two valence electrons highlight the importance of the duet rule for the first electron shell and demonstrate the exceptions to the octet rule, which are crucial for fully understanding the diversity of chemical behavior among elements. This knowledge forms the cornerstone for advanced studies in chemistry, materials science, and many other scientific disciplines.
Latest Posts
Latest Posts
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does He Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
