How Many Valence Electrons In Iron
Muz Play
Mar 19, 2025 · 6 min read
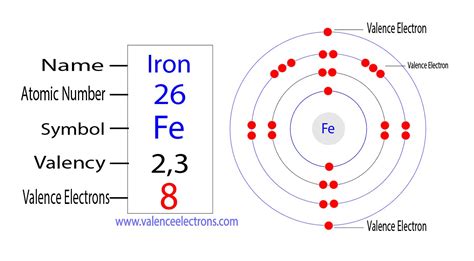
Table of Contents
How Many Valence Electrons Does Iron Have? A Deep Dive into Electronic Configuration
Iron, a ubiquitous element fundamental to life and industry, presents an intriguing case study in electron configuration. Understanding its valence electrons – those outermost electrons involved in chemical bonding – is crucial to comprehending its diverse chemical properties and its role in various processes. This article will delve into the intricacies of iron's electronic structure, explaining how many valence electrons it possesses, why this number is significant, and how it impacts its behavior in different contexts.
Understanding Electron Configuration and Valence Electrons
Before we tackle iron specifically, let's establish a foundational understanding of electron configuration and valence electrons. Every atom is composed of a nucleus containing protons and neutrons, surrounded by orbiting electrons arranged in specific energy levels or shells. These shells are further subdivided into subshells (s, p, d, and f), each capable of holding a specific number of electrons.
The electron configuration describes the arrangement of electrons within these shells and subshells. It follows a set of rules, dictated by quantum mechanics, that dictates electron filling order. This order, generally following the Aufbau principle, determines the stability and reactivity of an atom.
Valence electrons are the electrons residing in the outermost shell, the valence shell. These electrons are the most loosely bound to the atom and, therefore, participate most readily in chemical bonding. They determine an atom's reactivity, the types of bonds it can form, and its overall chemical behavior. The number of valence electrons often dictates the number of bonds an atom can form.
Determining Iron's Electron Configuration
Iron (Fe) has an atomic number of 26, meaning it possesses 26 protons and, in its neutral state, 26 electrons. To determine its electron configuration, we follow the Aufbau principle and fill the subshells according to their energy levels:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
This configuration can also be represented in a shorthand notation using the noble gas configuration of Argon ([Ar]):
[Ar] 4s² 3d⁶
This representation simplifies the notation by replacing the filled inner shells ([Ar] representing 1s² 2s² 2p⁶ 3s² 3p⁶) with the symbol of the preceding noble gas.
How Many Valence Electrons Does Iron Possess?
The question of how many valence electrons iron has is slightly more nuanced than a simple count of electrons in the highest energy level. While the 4s² electrons are clearly in the outermost shell, the involvement of the 3d⁶ electrons in chemical bonding complicates matters.
In many cases, iron is considered to have two valence electrons. This is primarily because the 4s electrons are generally more easily removed or shared during chemical bonding than the 3d electrons. Reactions involving iron frequently involve the participation of these two 4s electrons. For example, in the formation of Fe²⁺ ion, it loses the two 4s electrons.
However, the situation is not always so straightforward. In some instances, iron can utilize its 3d electrons in bonding, especially in higher oxidation states like Fe³⁺ or in complex formations. This means that iron's effective number of valence electrons can vary depending on the specific chemical environment and the type of bonding involved. The 3d electrons, though not in the outermost shell, are sufficiently close in energy to the 4s electrons to participate in bonding under certain circumstances.
This variable valency is a significant factor contributing to iron's versatile chemistry and its ability to form various compounds and complexes with diverse properties.
The Significance of Iron's Valence Electrons
The varying number of valence electrons available in iron directly influences its crucial role in a vast array of processes:
1. Biological Significance:
- Hemoglobin and Myoglobin: Iron's ability to readily accept and donate electrons is pivotal to its function in hemoglobin and myoglobin, the oxygen-transporting proteins in our blood and muscles. The iron ion at the center of the heme group cycles between Fe²⁺ and Fe³⁺ states, facilitating oxygen binding and release. This involves the participation of its valence electrons.
- Cytochromes: Iron is a key component of cytochromes, electron-carrying proteins integral to cellular respiration, the process that generates energy in cells. This electron transfer relies on the availability of iron's valence electrons.
- Enzymes: Iron is present in many enzymes, acting as a cofactor in various biochemical reactions. Its ability to change its oxidation state, a direct consequence of its valence electrons, allows it to catalyze these reactions.
2. Industrial Applications:
- Steel Production: Iron's chemical properties, particularly its ability to form strong metallic bonds, make it essential for steel production. Its valence electrons contribute directly to these bonds, creating the strong and durable materials used in countless applications.
- Catalysis: Iron compounds are used extensively as catalysts in various industrial processes, such as the Haber-Bosch process for ammonia synthesis. The catalytic activity stems from the ability of iron to accept and donate electrons during reaction intermediates, a characteristic determined by its valence electron configuration.
- Pigments and Dyes: Iron compounds impart distinctive colors, leading to their use as pigments and dyes in various applications. The color properties are often related to the electronic transitions involving iron's valence electrons.
3. Environmental Impact:
- Rust Formation: Iron's reactivity, driven by its valence electrons, contributes to the formation of rust (iron oxide), a process that can have significant environmental and economic consequences.
- Redox Reactions in the Environment: Iron plays a vital role in various redox reactions in the environment, influencing nutrient cycling and the fate of pollutants. Its ability to participate in electron transfer is linked directly to its valence electrons.
Iron's Oxidation States and Valence Electrons
Iron exhibits multiple oxidation states, predominantly +2 (ferrous) and +3 (ferric), reflecting its ability to lose different numbers of electrons.
-
Fe²⁺ (Ferrous): In this state, iron loses two electrons, typically the 4s electrons. Its electron configuration becomes [Ar] 3d⁶. While the 3d electrons are not considered valence electrons in the simplest model, they do play a significant role in complex formation and reactivity.
-
Fe³⁺ (Ferric): In this state, iron loses three electrons, commonly the two 4s electrons and one 3d electron. Its electron configuration becomes [Ar] 3d⁵. This configuration exhibits its own unique stability, explaining the prevalence of Fe³⁺ in many compounds.
The ability of iron to adopt these different oxidation states reflects the relatively small energy difference between its 4s and 3d electrons, allowing for varying participation in bonding and electron transfer reactions.
Conclusion: A Versatile Element
The question of how many valence electrons iron possesses is not a simple one-size-fits-all answer. While often considered to have two valence electrons (from the 4s subshell), the contribution of 3d electrons to bonding in certain chemical environments complicates this picture. This versatility in electron participation is directly responsible for iron's unique and crucial role in biology, industry, and the environment. Its ability to readily lose and gain electrons, its multiple oxidation states, and the involvement of both 4s and 3d electrons in various bonding interactions, all stem from its intricate electronic structure. This deeper understanding of iron's electron configuration underscores its importance as one of the most versatile and vital elements in the world around us. The seemingly simple question of valence electrons ultimately reveals the complexities and profound implications of atomic structure and its impact on the properties and behaviors of elements.
Latest Posts
Latest Posts
-
Bond Where Electrons Are Shared Equally
Mar 19, 2025
-
Is Formal Charge The Same As Oxidation Number
Mar 19, 2025
-
How To Find A Limit From A Graph
Mar 19, 2025
-
Area Bounded By Two Polar Curves
Mar 19, 2025
-
Glucose And Fructose Combine To Form
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
