Glucose And Fructose Combine To Form
Muz Play
Mar 19, 2025 · 7 min read
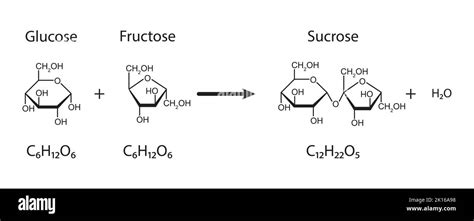
Table of Contents
Glucose and Fructose Combine to Form: Sucrose, a Deep Dive into the Chemistry and Biology of Table Sugar
Sucrose, the common table sugar we use daily, isn't just a sweetening agent; it's a fascinating molecule formed from the union of two simpler sugars: glucose and fructose. This article delves deep into the chemistry of this combination, its biological significance, its metabolism within our bodies, and its implications for health. We will explore the process of sucrose formation, its properties, and the diverse roles it plays in the natural world and in human society.
The Chemistry of Sucrose Formation: A Delicate Dance of Bonds
Glucose and fructose, both hexose sugars (meaning they contain six carbon atoms), combine through a process called dehydration synthesis or condensation. This reaction involves the removal of a water molecule (H₂O) as the two sugars link together. Specifically, the hydroxyl (-OH) group on carbon atom 1 of glucose reacts with the hydroxyl group on carbon atom 2 of fructose. This reaction forms a glycosidic bond, a type of covalent bond that connects the two sugar molecules. The resulting molecule, sucrose, is a disaccharide—a sugar composed of two monosaccharide units.
The Glycosidic Bond: The Key to Sucrose's Structure
The glycosidic bond in sucrose is an α-1, β-2-glycosidic linkage. This specific designation indicates:
- α (alpha): The configuration of the carbon atom 1 in glucose.
- 1: The carbon atom involved in the bond formation in glucose.
- β (beta): The configuration of the carbon atom 2 in fructose.
- 2: The carbon atom involved in the bond formation in fructose.
This seemingly minor detail is crucial. The specific configuration and location of the glycosidic bond dictate the three-dimensional structure of sucrose, its solubility in water, and how it interacts with enzymes in the body.
Understanding the Properties of Sucrose: Sweetness, Solubility, and More
Sucrose’s properties are directly linked to its chemical structure. Its sweetness is a sensory experience, but the molecular structure contributes to it. The arrangement of hydroxyl groups and the overall shape of the molecule interact with taste receptors on our tongues to produce the sensation of sweetness.
Sucrose’s high solubility in water is also a consequence of its many hydroxyl groups, which can form hydrogen bonds with water molecules. This high solubility makes it easily dissolved and usable in various food and beverage applications.
The Biological Significance of Sucrose: Nature's Sweet Energy
Sucrose is the primary form of sugar transported in plants. Plants synthesize sucrose through photosynthesis, using sunlight to convert carbon dioxide and water into glucose. This glucose is then converted into sucrose for efficient transport throughout the plant via the phloem, a specialized vascular tissue. Sucrose serves as a crucial energy source for plant growth and development. It is stored in various parts of the plant, such as roots, fruits, and seeds, providing energy for germination and growth. The concentration of sucrose in plant sap varies depending on the species and environmental conditions. This variability has led to various methods of sugar extraction and refinement for human consumption throughout history.
Sucrose in Fruits and Vegetables: A Natural Energy Source
Many fruits and vegetables contain naturally occurring sucrose. The sweetness of fruits is largely attributable to their sucrose content. This natural sucrose provides a readily available energy source for animals, including humans, who consume these fruits and vegetables. The role of sucrose in plant reproduction is also significant, as its presence in fruits attracts animals, facilitating seed dispersal.
Sucrose Metabolism in Humans: Digestion and Energy Production
When humans consume sucrose, it undergoes digestion in the small intestine. The enzyme sucrase, located on the surface of the intestinal cells, breaks down sucrose into its constituent monosaccharides: glucose and fructose. This process is called hydrolysis, the reverse of dehydration synthesis. A water molecule is added to break the glycosidic bond, releasing glucose and fructose, which are then absorbed into the bloodstream.
Glucose and Fructose: Different Metabolic Pathways
Glucose and fructose, while both providing energy, are metabolized differently. Glucose is directly utilized in cellular respiration to produce ATP (adenosine triphosphate), the body's primary energy currency. Fructose, however, primarily undergoes metabolism in the liver. Excess fructose consumption can lead to increased fat synthesis in the liver, potentially contributing to metabolic disorders such as non-alcoholic fatty liver disease.
The Role of Insulin in Glucose Metabolism
The metabolism of glucose is tightly regulated by the hormone insulin, secreted by the pancreas. Insulin facilitates the uptake of glucose from the bloodstream into cells, ensuring that glucose is available for energy production and preventing blood glucose levels from becoming excessively high.
Sucrose and Health: Balancing Sweetness with Moderation
While sucrose provides readily available energy, excessive consumption can have negative health consequences. High sucrose intake is linked to:
- Weight gain and obesity: Excess calories from sucrose are stored as fat if not used for energy.
- Type 2 diabetes: Chronic high blood sugar levels can lead to insulin resistance and type 2 diabetes.
- Dental caries (tooth decay): Bacteria in the mouth metabolize sucrose, producing acids that erode tooth enamel.
- Cardiovascular disease: High sucrose consumption is associated with increased risk factors for cardiovascular disease, such as high triglycerides and low HDL cholesterol.
- Non-alcoholic fatty liver disease (NAFLD): As mentioned earlier, excessive fructose metabolism in the liver can contribute to NAFLD.
Moderation and Healthy Alternatives: Making Informed Choices
The key to incorporating sucrose into a healthy diet is moderation. It is important to limit added sugars, including sucrose, in processed foods and beverages. Opting for natural sources of sweetness, such as fruits and vegetables, provides additional nutritional benefits and reduces the risk of consuming excessive amounts of refined sugar. Artificial sweeteners are another option, although they are also a subject of ongoing research and debate regarding their long-term health effects.
The Industrial Production of Sucrose: From Sugarcane and Sugar Beet to Table Sugar
Sucrose is primarily obtained from two sources: sugarcane and sugar beet. Sugarcane is a tropical plant that accumulates high levels of sucrose in its stalks. Sugar beet is a root vegetable cultivated in temperate climates that is also rich in sucrose. The extraction process involves multiple steps:
- Harvesting: The sugarcane or sugar beet is harvested and transported to processing facilities.
- Extraction: The sucrose is extracted from the plant material using either mechanical pressing (for sugarcane) or diffusion (for sugar beet).
- Purification: The raw sugar juice undergoes purification steps to remove impurities and non-sugar components.
- Crystallization: The purified sugar solution is concentrated and then cooled to allow sucrose crystals to form.
- Centrifugation: The sucrose crystals are separated from the remaining liquid (molasses).
- Drying: The sucrose crystals are dried to remove any remaining moisture.
This process yields refined white sugar, which is the sucrose we commonly use in our kitchens. Depending on the refining process, different types of sugar can be produced, such as brown sugar, which retains some molasses, offering a richer flavor and slightly different nutritional profile.
Beyond Sweetness: The Diverse Applications of Sucrose
Sucrose's versatility extends beyond its use as a sweetener. It plays a vital role in various industrial applications, including:
- Food preservation: Sucrose's high osmotic pressure inhibits microbial growth, making it an effective preservative in jams, jellies, and other food products.
- Pharmaceuticals: Sucrose is used as a filler and excipient in many pharmaceutical formulations, improving the texture and stability of medicines.
- Cosmetics: Sucrose derivatives are used in some cosmetics as humectants, helping to retain moisture in the skin.
- Biotechnology: Sucrose serves as a carbon source in various biotechnological applications, supporting the growth of microorganisms.
Conclusion: Sucrose – A Complex Molecule with Far-Reaching Implications
Sucrose, the seemingly simple table sugar, reveals a complex interplay of chemistry, biology, and industrial processes. Understanding its formation, metabolism, and health implications empowers us to make informed choices about our diet and consumption habits. While enjoying the sweetness of sucrose, it’s crucial to remember the importance of moderation and the benefits of consuming it in its natural forms within a balanced diet. Further research continues to unravel the intricate details of sucrose’s roles in our bodies and the environment, ensuring its relevance will remain a key area of study for years to come.
Latest Posts
Latest Posts
-
Strong Acid And Strong Base Reaction
Mar 19, 2025
-
What Is The Period Of A Cosine Function
Mar 19, 2025
-
Elements In An Element Family Have Similar
Mar 19, 2025
-
Which Macromolecule Is Not A Polymer
Mar 19, 2025
-
The Cells Of This Tissue Shorten To Exert Force
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Glucose And Fructose Combine To Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
