Which Macromolecule Is Not A Polymer
Muz Play
Mar 19, 2025 · 5 min read
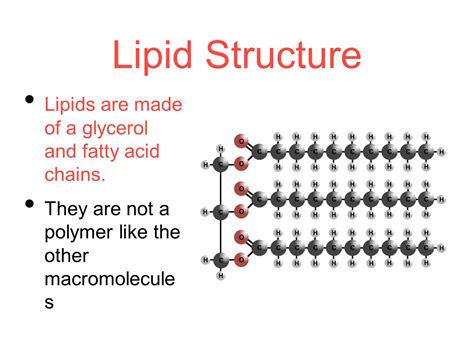
Table of Contents
Which Macromolecule Is Not a Polymer?
Macromolecules are giant molecules, essential for life, built from smaller subunits. While many macromolecules are polymers – long chains of repeating subunits called monomers – one crucial class stands apart: lipids. This article delves into the fascinating world of macromolecules, focusing specifically on why lipids are the exception to the polymer rule, exploring their diverse structures and vital biological functions.
Understanding Macromolecules and Polymers
Before we pinpoint the non-polymer macromolecule, let's establish a clear understanding of the basic terms. Macromolecules are large molecules that are essential for the structure and function of living organisms. They are generally categorized into four major groups:
-
Carbohydrates: These are composed of carbon, hydrogen, and oxygen atoms, often in a ratio of 1:2:1. They serve as a primary energy source and structural components in cells. Examples include starch, glycogen, and cellulose, all of which are polymers of glucose monomers.
-
Proteins: Proteins are built from amino acid monomers linked by peptide bonds. They have incredibly diverse functions, acting as enzymes, structural elements, hormones, and transporters, among many other roles. The sequence of amino acids determines the protein's unique three-dimensional structure and function, making them quintessential examples of polymers.
-
Nucleic Acids: These are the carriers of genetic information. DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) are polymers composed of nucleotide monomers, each containing a sugar, a phosphate group, and a nitrogenous base. The sequence of nucleotides dictates the genetic code.
-
Lipids: This is where the polymer rule breaks down. While lipids are large and biologically significant macromolecules, they are not typically considered polymers in the same way as carbohydrates, proteins, or nucleic acids. We will explore this in detail further below.
Why Lipids Aren't Polymers: A Detailed Examination
The key characteristic of a polymer is the repetitive linkage of identical or similar monomers. Carbohydrates, proteins, and nucleic acids all exhibit this linear or branched chain structure built from repeating units. Lipids, on the other hand, exhibit a far greater structural diversity. While some lipids might contain repeating units, they don't share the same consistent, chain-like structure defined by covalent bonds between identical monomers that characterize true polymers.
Let's examine the different types of lipids:
1. Triglycerides: Esterification, Not Polymerization
Triglycerides, the most common type of lipid, are composed of a glycerol molecule and three fatty acid molecules. The linkage between glycerol and fatty acids is through ester bonds, formed by a dehydration reaction. While this involves repetitive units (three fatty acids), it's not the same type of repetitive bonding found in classic polymers. The fatty acids themselves can vary greatly in length and saturation, further differentiating them from the more uniform structure of a true polymer. Therefore, triglycerides are more accurately described as assembled molecules rather than polymers.
2. Phospholipids: A Modified Triglyceride Structure
Phospholipids are similar to triglycerides, but one fatty acid is replaced by a phosphate group and often a polar head group. This structural modification is crucial for their role in forming cell membranes. While they contain repeating units (the fatty acids), the overall structure is not a simple repeating chain, and the connection between components isn’t the same type of linkage found in typical polymers.
3. Steroids: Complex Ring Structures
Steroids, such as cholesterol and sex hormones, are characterized by their four fused carbon rings. Their structure is far from linear and lacks the repetitive monomeric units characteristic of polymers. They are complex molecules built from isoprene units, but the arrangement and bonding are distinct from the linear or branched chains of polymers.
4. Waxes: Ester Linkage, but Not a Polymer
Waxes are esters formed between a long-chain alcohol and a long-chain fatty acid. Similar to triglycerides, the ester linkage isn't the same kind of repeating bond found in polymers. Although there is a pattern in the long chains, this structure isn't considered polymeric because the connections don’t build a linear chain of identical repeating units.
The Importance of Lipid Diversity
The fact that lipids are not polymers highlights their structural and functional diversity. This versatility is essential for their numerous biological roles, including:
-
Energy Storage: Triglycerides store large amounts of energy in a compact form.
-
Cell Membrane Structure: Phospholipids form the fundamental bilayer structure of cell membranes, regulating the passage of substances.
-
Hormone Production: Steroids act as crucial hormones, regulating a variety of physiological processes.
-
Insulation and Protection: Lipids provide insulation against temperature changes and cushion vital organs.
Distinguishing Features: Polymers vs. Lipids
Here's a table summarizing the key differences between polymers and lipids:
| Feature | Polymers (Carbohydrates, Proteins, Nucleic Acids) | Lipids |
|---|---|---|
| Monomers | Identical or similar repeating units | Variable, often with diverse structures |
| Bonding | Covalent bonds between monomers (e.g., glycosidic, peptide, phosphodiester) | Ester bonds (in triglycerides, phospholipids, and waxes), various other bonds in steroids |
| Structure | Typically linear or branched chains | Diverse; linear, branched, ring structures |
| Repetition | High degree of repetition of monomers | Less consistent repetition of units |
| Example | Starch, cellulose, proteins, DNA | Triglycerides, phospholipids, cholesterol |
The Significance of Classification
The distinction between polymers and lipids is not merely a matter of semantics. It reflects fundamental differences in their chemical structure, biosynthesis, and biological functions. Understanding this distinction is crucial for comprehending the complexity of biological systems. The classification scheme helps us organize and interpret the vast array of molecules found in living organisms, leading to a deeper understanding of their properties and interactions.
Conclusion: A Non-Polymer Macromolecule with Crucial Roles
In conclusion, while carbohydrates, proteins, and nucleic acids are all polymers built from repeating monomers, lipids stand apart as a major class of macromolecules that are not typically considered polymers. Their diverse structures, including triglycerides, phospholipids, steroids, and waxes, reflect their wide range of biological roles, crucial for energy storage, membrane structure, hormone production, and many other essential processes. The unique characteristics of lipids emphasize the incredible complexity and diversity of the molecular world that underpins all life. Understanding the differences between polymers and lipids is vital for a thorough understanding of biochemistry and cell biology.
Latest Posts
Latest Posts
-
Lewis Diagram For A Ion With A Total Of Electrons
Mar 19, 2025
-
A The Symbol For Sample Standard Deviation Is
Mar 19, 2025
-
What Are The Columns In The Periodic Table Called
Mar 19, 2025
-
How Is The Air Volume Affected By Temperature
Mar 19, 2025
-
Difference Between Voltaic Cell And Electrolytic Cell
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Macromolecule Is Not A Polymer . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
