How Much Atp Is Produced In Oxidative Phosphorylation
Muz Play
Mar 27, 2025 · 5 min read
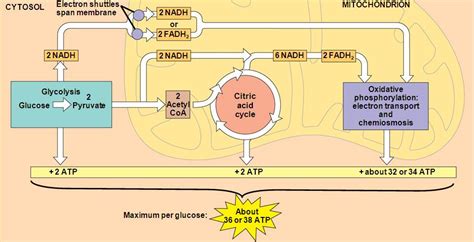
Table of Contents
How Much ATP is Produced in Oxidative Phosphorylation? A Deep Dive into Cellular Energy Production
Oxidative phosphorylation (OXPHOS) is the final and most significant stage of cellular respiration, responsible for generating the vast majority of ATP – the cell's primary energy currency. Understanding the precise amount of ATP produced is crucial for comprehending cellular metabolism and its implications for health and disease. However, the exact number isn't a simple, universally agreed-upon figure. This article will delve into the complexities of ATP production in OXPHOS, exploring the factors influencing yield and the nuances of its calculation.
The Electron Transport Chain: The Engine of Oxidative Phosphorylation
OXPHOS takes place within the inner mitochondrial membrane, harnessing the energy stored in reduced electron carriers, NADH and FADH2, generated during glycolysis and the citric acid cycle. These carriers donate their high-energy electrons to a series of protein complexes embedded in the inner mitochondrial membrane – the electron transport chain (ETC).
The Four Complexes of the ETC
The ETC comprises four major protein complexes (Complexes I-IV), each facilitating electron transfer and proton pumping.
- Complex I (NADH dehydrogenase): Accepts electrons from NADH and pumps protons (H+) across the inner mitochondrial membrane into the intermembrane space.
- Complex II (succinate dehydrogenase): Accepts electrons from FADH2, a slightly less energetic electron donor, and does not directly pump protons.
- Complex III (cytochrome bc1 complex): Receives electrons from Complex I or II and further pumps protons into the intermembrane space via the Q cycle.
- Complex IV (cytochrome c oxidase): The final complex, receiving electrons from Complex III and transferring them to molecular oxygen (O2), forming water (H2O). This step also contributes to proton pumping.
The movement of protons across the membrane establishes a proton motive force (PMF) – an electrochemical gradient with a higher concentration of protons in the intermembrane space compared to the mitochondrial matrix. This gradient stores the energy derived from electron transport.
Chemiosmosis: Harnessing the Proton Gradient
The PMF drives ATP synthesis through a process called chemiosmosis. Protons flow back into the mitochondrial matrix through a channel within ATP synthase (Complex V), a remarkable molecular turbine. This proton flow drives the rotation of a part of ATP synthase, catalyzing the phosphorylation of ADP to ATP.
The P/O Ratio: A Key Concept
The phosphorylation-to-oxygen ratio (P/O ratio) represents the number of ATP molecules synthesized per atom of oxygen reduced. This ratio is crucial for calculating the overall ATP yield of OXPHOS. Theoretically:
- NADH: A P/O ratio of approximately 2.5 is often cited for NADH, meaning each NADH molecule contributes to the synthesis of about 2.5 ATP molecules.
- FADH2: FADH2 has a lower P/O ratio, typically around 1.5, due to its entry point into the ETC at Complex II, bypassing Complex I's proton pumping.
Calculating the Total ATP Yield: The Nuances
Calculating the total ATP yield from OXPHOS requires considering several factors:
- The number of NADH and FADH2 molecules produced: The amount varies depending on the substrate being oxidized (e.g., glucose, fatty acids).
- The efficiency of the ETC and ATP synthase: The actual P/O ratio can deviate from the theoretical values due to proton leaks across the inner mitochondrial membrane and variations in the efficiency of ATP synthesis.
- The use of the glycerol-3-phosphate shuttle: Some cells utilize the glycerol-3-phosphate shuttle, a system that transfers reducing equivalents from cytoplasmic NADH to FADH2 in the mitochondria, resulting in a lower ATP yield per NADH molecule.
- The malate-aspartate shuttle: This shuttle system allows for the transfer of reducing equivalents from cytosolic NADH to mitochondrial NADH, resulting in a higher ATP yield than the glycerol-3-phosphate shuttle.
Variations and Complicating Factors
The previously mentioned theoretical values (2.5 ATP per NADH and 1.5 ATP per FADH2) are simplified representations. The actual ATP yield can fluctuate depending on several factors including:
- Temperature: Changes in temperature can affect the efficiency of the ETC and ATP synthase.
- pH: Optimal pH is necessary for the proper function of the enzymes involved in oxidative phosphorylation.
- Membrane potential: The precise level of proton gradient across the inner mitochondrial membrane plays a significant role in ATP synthesis.
- Substrate availability: Efficient ATP production requires a sufficient supply of substrates.
- Inhibitors and uncouplers: Substances that interfere with the ETC or ATP synthase can dramatically reduce ATP production.
The Glucose Example: An In-Depth Look
Let's consider the complete oxidation of one glucose molecule as a case study:
- Glycolysis: Yields 2 ATP (net), 2 NADH (cytosolic).
- Pyruvate Oxidation: Produces 2 NADH (mitochondrial) and 2 CO2 per glucose.
- Citric Acid Cycle: Yields 2 ATP, 6 NADH, 2 FADH2, and 4 CO2 per glucose.
Using the theoretical P/O ratios and considering the shuttle mechanisms:
- Cytosolic NADH (Glycolysis): Assuming the malate-aspartate shuttle (which is more efficient than the glycerol-3-phosphate shuttle), 2 NADH x 2.5 ATP/NADH = 5 ATP
- Mitochondrial NADH: (2 from pyruvate oxidation + 6 from citric acid cycle) = 8 NADH x 2.5 ATP/NADH = 20 ATP
- FADH2: 2 FADH2 x 1.5 ATP/FADH2 = 3 ATP
Therefore, the total ATP yield from OXPHOS is theoretically 5 + 20 + 3 = 28 ATP. Adding the 2 ATP from glycolysis and 2 ATP from the citric acid cycle, the total theoretical yield from the complete oxidation of one glucose molecule is approximately 32 ATP.
However, it's crucial to reiterate that this is a theoretical maximum. The actual yield is likely somewhat lower due to the factors mentioned earlier. Estimates often range from 28 to 32 ATP per glucose molecule, with 30 ATP being a common approximation.
Conclusion: The Dynamic Nature of ATP Production
The precise amount of ATP produced in oxidative phosphorylation is not a fixed number. It's a dynamic process influenced by numerous factors, including substrate availability, the efficiency of the ETC and ATP synthase, and the use of different shuttle systems. While simplified calculations often yield a theoretical maximum, the actual ATP yield is generally lower, making accurate quantification challenging. Understanding these complexities is essential for a comprehensive grasp of cellular energy metabolism and its vital role in biological processes. Future research will undoubtedly further refine our understanding of this intricate and crucial cellular pathway.
Latest Posts
Latest Posts
-
Adding Integers With The Same Sign
Mar 30, 2025
-
How Do You Divide Fractions With Exponents
Mar 30, 2025
-
N Type Semiconductor Vs P Type Semiconductor
Mar 30, 2025
-
Bipolar Junction Transistor As A Switch
Mar 30, 2025
-
Starch Glycogen And Cellulose Are Examples Of
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Much Atp Is Produced In Oxidative Phosphorylation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
