Starch Glycogen And Cellulose Are Examples Of
Muz Play
Mar 30, 2025 · 5 min read
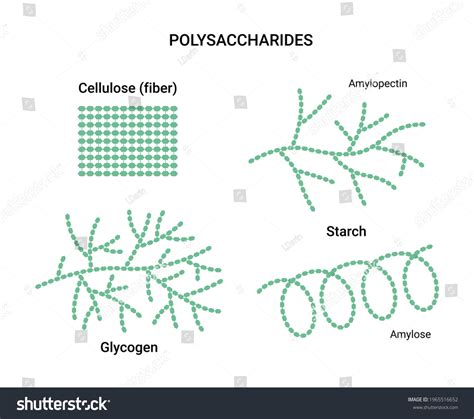
Table of Contents
Starch, Glycogen, and Cellulose: Examples of Polysaccharides and Their Diverse Roles
Starch, glycogen, and cellulose are all examples of polysaccharides, a crucial class of carbohydrates playing vital roles in living organisms. Understanding their structures and functions is key to comprehending fundamental biological processes. While all three are composed of glucose units, their differing structures lead to vastly different properties and biological roles. This article will delve into the specifics of each, exploring their chemical structures, biological functions, and the significance of their structural variations.
What are Polysaccharides?
Before delving into the specifics of starch, glycogen, and cellulose, let's establish a foundational understanding of polysaccharides. Polysaccharides are large, complex carbohydrates composed of long chains of monosaccharides (simple sugars) linked together by glycosidic bonds. These chains can be linear or branched, and the type of glycosidic bond and the arrangement of the monosaccharides significantly impact the polysaccharide's properties. Polysaccharides serve diverse roles in living organisms, including:
- Energy Storage: Some polysaccharides, like starch and glycogen, act as energy reserves, storing glucose for later use.
- Structural Support: Others, such as cellulose and chitin, provide structural support to cells and tissues.
- Cellular Recognition: Certain polysaccharides are involved in cell-cell recognition and communication.
Starch: The Plant's Energy Store
Starch is the primary energy storage polysaccharide in plants. It's found in various plant parts, particularly seeds, roots, and tubers. The glucose units in starch are linked primarily through α-1,4-glycosidic bonds, creating long chains. Starch exists in two main forms:
Amylose: A Linear Chain
Amylose is a linear polysaccharide consisting of several hundred to a thousand glucose units linked by α-1,4-glycosidic bonds. This linear structure results in a helical conformation. This helical structure is crucial for its compact storage and efficient breakdown when energy is needed. The tightly packed helix minimizes space occupation while allowing for easy access to the glucose units during enzymatic hydrolysis.
Amylopectin: A Branched Chain
Amylopectin, on the other hand, is a highly branched polysaccharide. While the majority of glucose units are linked by α-1,4-glycosidic bonds, branches occur approximately every 24-30 glucose residues through α-1,6-glycosidic bonds. These branches create a more compact structure than amylose, enhancing its efficiency as an energy storage molecule. The branches provide numerous points of access for enzymes to break down the polysaccharide into glucose molecules during respiration.
Glycogen: The Animal's Energy Reserve
Glycogen is the primary energy storage polysaccharide in animals and fungi. Structurally similar to amylopectin, glycogen is also composed of glucose units linked by α-1,4-glycosidic bonds, with branches formed by α-1,6-glycosidic bonds. However, glycogen has a higher degree of branching than amylopectin, with branches occurring every 8-12 glucose residues. This increased branching allows for rapid mobilization of glucose units when energy is required. Glycogen is stored primarily in the liver and muscles, acting as a readily available energy source for cellular processes. The highly branched structure ensures that a large number of glucose units can be released simultaneously, providing a quick energy boost. This rapid release is particularly important for activities requiring bursts of energy, like sprinting or fighting off a predator.
Glycogen's Role in Blood Sugar Regulation
The liver's glycogen stores play a vital role in maintaining blood glucose homeostasis. When blood sugar levels drop, the liver releases glucose from its glycogen stores through glycogenolysis, a process involving enzymatic breakdown of glycogen. This process helps to maintain stable blood glucose levels, crucial for the proper functioning of the brain and other organs.
Cellulose: The Structural Backbone of Plants
Cellulose, unlike starch and glycogen, is a structural polysaccharide that provides rigidity and support to plant cell walls. It's the most abundant organic polymer on Earth. Cellulose is composed of glucose units linked by β-1,4-glycosidic bonds. This seemingly small difference in the glycosidic bond configuration—β instead of α—has profound consequences for its structure and function.
Linear Structure and Hydrogen Bonding
The β-1,4-glycosidic bonds in cellulose result in a linear, unbranched structure. These linear chains aggregate together through hydrogen bonding to form strong microfibrils, which further assemble into macrofibrils, providing significant tensile strength to the plant cell wall. This structure is crucial for the plant's ability to withstand various physical stresses and maintain its shape. The hydrogen bonds between adjacent cellulose chains contribute significantly to the overall strength and rigidity of the plant cell wall. This robust structure is essential for providing support to the plant and protecting it from mechanical damage.
Digestibility: The Role of β-1,4-glycosidic Bonds
The β-1,4-glycosidic bonds in cellulose are indigestible to most animals, including humans. Humans lack the necessary enzymes, such as cellulase, to break down these bonds. However, some animals, such as herbivores, have symbiotic relationships with microorganisms in their digestive tracts that produce cellulase, allowing them to digest cellulose and obtain energy from it. These microorganisms play a vital role in the breakdown of cellulose, making it a usable energy source for herbivores.
Comparison Table: Starch, Glycogen, and Cellulose
| Feature | Starch | Glycogen | Cellulose |
|---|---|---|---|
| Monomer | Glucose | Glucose | Glucose |
| Glycosidic Bond | α-1,4 (primarily), α-1,6 (branches) | α-1,4 (primarily), α-1,6 (branches) | β-1,4 |
| Structure | Linear (amylose), branched (amylopectin) | Highly branched | Linear, unbranched |
| Function | Energy storage in plants | Energy storage in animals and fungi | Structural support in plants |
| Digestibility | Digestible by animals | Digestible by animals | Indigestible by most animals |
Conclusion: The Importance of Structural Differences
Starch, glycogen, and cellulose, despite being composed of the same basic monomer—glucose—demonstrate the profound impact of structural variations on biological function. The subtle differences in glycosidic bond configurations and branching patterns result in vastly different properties, adapting these polysaccharides to their respective roles in energy storage and structural support within living organisms. Understanding these differences is crucial for appreciating the complexity and efficiency of biological systems. Further research into these polysaccharides continues to reveal insights into potential applications in various fields, including biofuels, biomaterials, and medicine. The ongoing exploration of these vital molecules promises to unlock further understanding of biological processes and inspire innovative applications in the future.
Latest Posts
Latest Posts
-
Are Temperature And Volume Directly Proportional
Apr 01, 2025
-
Is Hydrogen More Electronegative Than Oxygen
Apr 01, 2025
-
What Happens When An Atom Gains Or Loses An Electron
Apr 01, 2025
-
How To Name Ionic And Covalent Bonds
Apr 01, 2025
-
Liquid In A Liquid Solution Example
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Starch Glycogen And Cellulose Are Examples Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
