How To Name Ionic And Covalent Bonds
Muz Play
Apr 01, 2025 · 6 min read
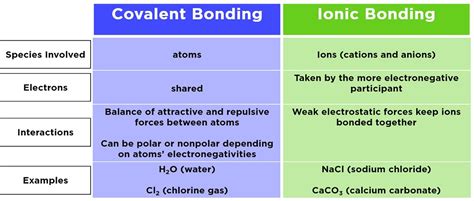
Table of Contents
How to Name Ionic and Covalent Bonds: A Comprehensive Guide
Naming chemical compounds might seem daunting at first, but with a structured approach, it becomes a manageable and even enjoyable skill. This comprehensive guide delves into the intricacies of naming ionic and covalent compounds, equipping you with the knowledge to confidently tackle this fundamental aspect of chemistry. We will explore the rules, exceptions, and strategies for accurately naming a wide range of compounds.
Understanding the Fundamentals: Ionic vs. Covalent Bonds
Before diving into the naming conventions, it's crucial to understand the fundamental difference between ionic and covalent bonds. This distinction dictates the naming system used.
Ionic Bonds: A Transfer of Electrons
Ionic bonds form between metals and nonmetals. The metal atom donates one or more electrons to the nonmetal atom, creating positively charged cations (metal ions) and negatively charged anions (nonmetal ions). This electrostatic attraction between oppositely charged ions constitutes the ionic bond. Think of it as a transfer of ownership – electrons completely leave one atom and become part of another.
Examples: NaCl (sodium chloride), MgO (magnesium oxide), CaCl₂ (calcium chloride).
Covalent Bonds: Shared Electrons
Covalent bonds, on the other hand, form between nonmetals. Instead of transferring electrons, atoms share electrons to achieve a stable electron configuration (usually a full outer shell). This shared electron pair constitutes the covalent bond. Think of it as a shared resource – both atoms have access to the electrons.
Examples: H₂O (water), CO₂ (carbon dioxide), CH₄ (methane).
Naming Ionic Compounds: A Step-by-Step Guide
The naming of ionic compounds follows a systematic approach:
-
Identify the cation (positive ion): The cation is named first, using the element's name. If the cation is a transition metal (elements in the d-block of the periodic table) that can have multiple charges, its charge must be specified using Roman numerals in parentheses.
-
Identify the anion (negative ion): The anion is named second. For monatomic anions (single-atom ions), the name ends in "-ide."
-
Combine the names: The cation name is followed by the anion name.
Let's illustrate with examples:
- NaCl (Sodium Chloride): Sodium (Na⁺) is the cation, and chloride (Cl⁻) is the anion.
- MgO (Magnesium Oxide): Magnesium (Mg²⁺) is the cation, and oxide (O²⁻) is the anion.
- FeCl₃ (Iron(III) Chloride): Iron (Fe) can have multiple charges (+2 or +3). In FeCl₃, iron has a +3 charge, hence Iron(III). Chloride (Cl⁻) is the anion.
- Cu₂O (Copper(I) Oxide): Copper (Cu) can have a +1 or +2 charge. In Cu₂O, copper has a +1 charge.
Dealing with Polyatomic Ions:
Polyatomic ions are groups of atoms that carry a net charge. These ions have specific names that you need to memorize. Some common polyatomic anions include:
- Nitrate (NO₃⁻): Found in compounds like KNO₃ (potassium nitrate).
- Sulfate (SO₄²⁻): Found in compounds like Na₂SO₄ (sodium sulfate).
- Phosphate (PO₄³⁻): Found in compounds like Ca₃(PO₄)₂ (calcium phosphate).
- Hydroxide (OH⁻): Found in compounds like NaOH (sodium hydroxide).
- Carbonate (CO₃²⁻): Found in compounds like CaCO₃ (calcium carbonate).
- Ammonium (NH₄⁺): This is a polyatomic cation. It's found in compounds like NH₄Cl (ammonium chloride).
When naming compounds containing polyatomic ions, simply use the name of the cation followed by the name of the polyatomic anion.
Example: (NH₄)₂SO₄ is named Ammonium Sulfate.
Naming Covalent Compounds: A Different Approach
Naming covalent compounds follows a different set of rules than ionic compounds. The key difference lies in using prefixes to indicate the number of atoms of each element present in the molecule.
-
Identify the less electronegative element: This element is named first. Electronegativity is a measure of an atom's ability to attract electrons in a chemical bond. Generally, electronegativity increases across a period and decreases down a group in the periodic table.
-
Identify the more electronegative element: This element is named second, with its name ending in "-ide."
-
Use prefixes: Prefixes indicate the number of atoms of each element. These prefixes include:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
Note: the prefix "mono-" is usually omitted for the first element unless it's needed to distinguish between different compounds (e.g., CO vs. CO₂).
Let's look at some examples:
- CO (Carbon Monoxide): Carbon is less electronegative than oxygen. There's one carbon atom and one oxygen atom.
- CO₂ (Carbon Dioxide): One carbon atom and two oxygen atoms.
- N₂O₄ (Dinitrogen Tetroxide): Two nitrogen atoms and four oxygen atoms.
- PCl₅ (Phosphorus Pentachloride): One phosphorus atom and five chlorine atoms.
- SF₆ (Sulfur Hexafluoride): One sulfur atom and six fluorine atoms.
Exceptions and Special Cases:
While the rules provide a solid framework, some exceptions and special cases exist:
-
Acids: Acids are covalent compounds that release hydrogen ions (H⁺) when dissolved in water. Their naming conventions differ slightly. For example, HCl (hydrogen chloride) is called hydrochloric acid in aqueous solution.
-
Binary Acids: These acids contain only hydrogen and another nonmetal. Their names begin with “hydro-” followed by the nonmetal root name with the suffix "-ic acid." For example, HBr is hydrobromic acid.
-
Oxoacids: Oxoacids contain hydrogen, oxygen, and another nonmetal. Their naming is more complex, with different suffixes depending on the oxidation state of the central nonmetal atom.
-
Hydrates: Hydrates are compounds that incorporate water molecules into their crystal structure. Their names include a prefix indicating the number of water molecules, followed by "hydrate." For example, CuSO₄·5H₂O is copper(II) sulfate pentahydrate.
Tips and Tricks for Mastering Chemical Nomenclature:
- Practice, practice, practice: The more you practice, the more comfortable you'll become with naming compounds.
- Use flashcards or mnemonic devices: This can be helpful for memorizing polyatomic ion names.
- Understand the underlying principles: Understanding the difference between ionic and covalent bonding is crucial for applying the correct naming conventions.
- Consult a reliable reference: Use a chemistry textbook or online resource to verify your answers.
Advanced Topics in Chemical Nomenclature
Beyond the basic principles, the field of chemical nomenclature extends to more complex situations, encompassing organic chemistry, coordination compounds, and more specialized naming conventions.
-
Organic Compounds: Organic chemistry deals with carbon-based compounds, which have their own extensive set of naming rules (IUPAC nomenclature). This involves identifying functional groups and using a system of prefixes, suffixes, and numbers to describe the molecule's structure.
-
Coordination Compounds: Coordination compounds involve a central metal atom surrounded by ligands (molecules or ions). Naming these compounds requires understanding oxidation states, ligand names, and prefixes indicating the number of each ligand.
Mastering chemical nomenclature requires consistent effort, but it's a rewarding journey that unlocks a deeper understanding of the structure and properties of chemical compounds. By diligently following the guidelines, practicing regularly, and consulting reliable resources, you can become proficient in naming both ionic and covalent compounds with confidence.
Latest Posts
Latest Posts
-
Write An Equation Of The Line In Standard Form
Apr 02, 2025
-
How Does Carbon Dioxide Enter The Leaf
Apr 02, 2025
-
Arrhenius Acid Vs Bronsted Lowry Acid
Apr 02, 2025
-
Double And Half Angle Identities Worksheet
Apr 02, 2025
-
What Color Is A Animal Cell
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Name Ionic And Covalent Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
