How To Calculate E Not Cell
Muz Play
Mar 16, 2025 · 6 min read
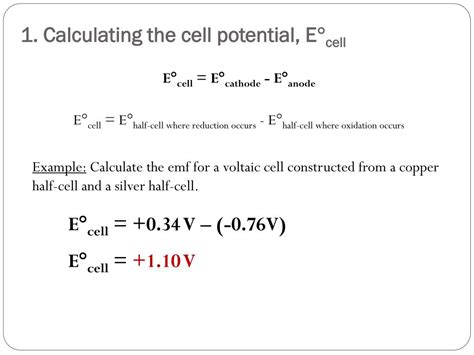
Table of Contents
How to Calculate E°cell: A Comprehensive Guide
Calculating the standard cell potential, E°cell, is crucial in electrochemistry for predicting the spontaneity and determining the equilibrium constant of a redox reaction. This comprehensive guide will walk you through the process, covering various aspects, from understanding fundamental concepts to tackling complex scenarios. We'll explore different methods and provide practical examples to solidify your understanding.
Understanding the Fundamentals: What is E°cell?
The standard cell potential, denoted as E°cell (or sometimes E°), represents the difference in electrical potential between two half-cells under standard conditions. These standard conditions are typically defined as 298 K (25°C), 1 atm pressure, and 1 M concentration for all aqueous species. A positive E°cell indicates a spontaneous reaction (Gibbs Free Energy, ΔG < 0), while a negative E°cell indicates a non-spontaneous reaction (ΔG > 0). Understanding this relationship is key to utilizing E°cell calculations.
Key Concepts:
- Half-cell reactions: Redox reactions are composed of two half-reactions: an oxidation half-reaction (loss of electrons) and a reduction half-reaction (gain of electrons). Each half-reaction has its own standard reduction potential (E°red).
- Standard Reduction Potentials (E°red): These values represent the tendency of a species to gain electrons under standard conditions. They are tabulated and readily available in electrochemical data tables. A more positive E°red indicates a stronger tendency to be reduced.
- Nernst Equation: While E°cell deals with standard conditions, the Nernst equation allows you to calculate the cell potential under non-standard conditions (different concentrations and temperatures).
Calculating E°cell: The Standard Approach
The most straightforward method for calculating E°cell involves using the standard reduction potentials of the individual half-reactions. The calculation follows these steps:
-
Identify the half-reactions: Deconstruct the overall redox reaction into its oxidation and reduction half-reactions.
-
Find the standard reduction potentials: Consult a standard reduction potential table (available in most chemistry textbooks and online resources) to find the E°red values for both half-reactions.
-
Reverse the oxidation half-reaction: The standard reduction potential table provides values for reduction half-reactions. If a half-reaction appears as an oxidation in your overall reaction, you must reverse it. Remember that reversing the reaction also changes the sign of the E°red.
-
Calculate E°cell: The standard cell potential is calculated by subtracting the standard reduction potential of the oxidation half-reaction from the standard reduction potential of the reduction half-reaction:
E°cell = E°red (reduction) - E°red (oxidation)
Example:
Let's calculate E°cell for the following reaction:
Zn(s) + Cu²⁺(aq) → Zn²⁺(aq) + Cu(s)
-
Half-reactions:
Oxidation: Zn(s) → Zn²⁺(aq) + 2e⁻ Reduction: Cu²⁺(aq) + 2e⁻ → Cu(s)
-
Standard Reduction Potentials:
E°red (Zn²⁺/Zn) = -0.76 V E°red (Cu²⁺/Cu) = +0.34 V
-
Reverse the Oxidation: The Zn half-reaction is an oxidation. Reversing it doesn't change its numerical value but changes the sign:
E°red (Zn/Zn²⁺) = +0.76 V (Note the sign change)
-
Calculate E°cell:
E°cell = E°red (Cu²⁺/Cu) - E°red (Zn/Zn²⁺) = 0.34 V - (-0.76 V) = +1.10 V
Since E°cell is positive, this reaction is spontaneous under standard conditions.
Dealing with Non-Standard Conditions: The Nernst Equation
The Nernst equation is essential when dealing with conditions deviating from standard state (1 M concentration, 1 atm pressure, 25°C). It accounts for the impact of concentration on the cell potential:
Ecell = E°cell - (RT/nF)lnQ
Where:
- Ecell: Cell potential under non-standard conditions.
- E°cell: Standard cell potential.
- R: Ideal gas constant (8.314 J/mol·K)
- T: Temperature in Kelvin.
- n: Number of moles of electrons transferred in the balanced redox reaction.
- F: Faraday's constant (96,485 C/mol)
- Q: Reaction quotient, representing the ratio of products to reactants at non-standard conditions.
Simplifying the Nernst Equation at 25°C:
At 25°C (298 K), the Nernst equation can be simplified to:
Ecell = E°cell - (0.0592/n)logQ
Example using the Nernst Equation:
Let's consider the same Zn/Cu cell, but now with [Zn²⁺] = 0.1 M and [Cu²⁺] = 1.0 M at 25°C.
-
Calculate Q: The reaction quotient is:
Q = [Zn²⁺]/[Cu²⁺] = 0.1 M / 1.0 M = 0.1
-
Determine n: Two electrons are transferred in the balanced reaction (n = 2).
-
Apply the simplified Nernst equation:
Ecell = E°cell - (0.0592/n)logQ = 1.10 V - (0.0592/2)log(0.1) = 1.10 V - (-0.0296 V) = 1.13 V
The cell potential under these non-standard conditions is 1.13 V, slightly higher than the standard cell potential.
Advanced Scenarios and Considerations
1. Multiple Half-Reactions: If your overall reaction involves more than one oxidation or reduction half-reaction, you need to carefully balance the electrons transferred before calculating E°cell. Make sure the number of electrons lost in oxidation equals the number of electrons gained in reduction.
2. Concentration Cells: Concentration cells consist of two half-cells with the same electrodes but different concentrations of the electrolyte. The cell potential arises solely from the concentration difference. The Nernst equation is crucial for calculating the potential of concentration cells.
3. Temperature Dependence: While the simplified Nernst equation is convenient for 25°C, remember to use the full Nernst equation for calculations at other temperatures. The temperature dependence of E°cell itself is relatively small but can be significant in precise measurements.
4. Activity vs. Concentration: The Nernst equation strictly uses activities instead of concentrations. However, at low concentrations, activity and concentration are approximately equal. At higher concentrations, activity coefficients must be considered for greater accuracy.
Practical Applications of E°cell Calculations
The ability to calculate E°cell has widespread applications in various fields:
-
Corrosion Prediction: E°cell calculations help predict the susceptibility of metals to corrosion. A positive E°cell for a metal-electrolyte system indicates a tendency for corrosion.
-
Battery Design: E°cell calculations are crucial in designing and optimizing batteries. A higher E°cell indicates a higher voltage output for a battery.
-
Electroplating: The potential needed for effective electroplating can be determined using E°cell and the Nernst equation.
-
Environmental Monitoring: E°cell measurements can be used to monitor redox reactions in environmental systems, providing insights into water quality and pollution levels.
Conclusion
Calculating E°cell is a fundamental skill in electrochemistry with far-reaching implications. Mastering this calculation, along with understanding the Nernst equation and its implications, allows for the prediction and manipulation of redox reactions under various conditions. This detailed guide provides a robust foundation for tackling diverse electrochemical problems and further exploration of this fascinating field. Remember to always consult reliable sources for standard reduction potentials and carefully consider the specific conditions of your electrochemical system.
Latest Posts
Latest Posts
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
-
Why Do Ions Travel Back And Forth In Orbitrap
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate E Not Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
