How To Calculate The Ph At The Equivalence Point
Muz Play
Mar 24, 2025 · 6 min read
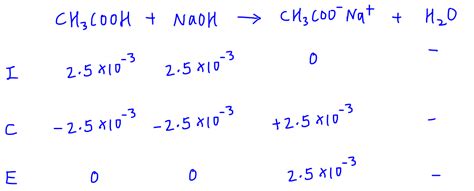
Table of Contents
How to Calculate the pH at the Equivalence Point
Calculating the pH at the equivalence point of a titration is crucial for understanding the titration curve and selecting appropriate indicators. The equivalence point represents the stoichiometric point where the moles of titrant added exactly equal the moles of analyte present. However, the pH at this point isn't always neutral (pH 7). It depends heavily on the nature of the acid and base involved in the titration. This comprehensive guide will walk you through various scenarios, providing step-by-step calculations and explanations.
Understanding the Equivalence Point
Before diving into the calculations, let's solidify our understanding of the equivalence point. It's the point in a titration where the added titrant has completely neutralized the analyte. This doesn't necessarily mean the resulting solution will be neutral (pH 7). The pH at the equivalence point depends on the strength of the acid and base involved:
-
Strong Acid - Strong Base Titration: The pH at the equivalence point is 7. This is because the resulting salt formed is neutral.
-
Strong Acid - Weak Base Titration: The pH at the equivalence point is less than 7 (acidic). The conjugate acid of the weak base remains in solution, lowering the pH.
-
Weak Acid - Strong Base Titration: The pH at the equivalence point is greater than 7 (basic). The conjugate base of the weak acid remains in solution, raising the pH.
-
Weak Acid - Weak Base Titration: The pH at the equivalence point is difficult to predict precisely and depends on the relative strengths of the acid and base. It's often close to 7 but not necessarily.
Calculating pH at the Equivalence Point: Different Scenarios
Let's delve into calculating the pH at the equivalence point for each scenario:
1. Strong Acid - Strong Base Titration
This is the simplest case. Since both the acid and base are strong, they completely dissociate. At the equivalence point, the solution contains only the salt formed and water. The salt will not significantly affect the pH, resulting in a neutral solution (pH 7). No further calculation is needed for a strong acid-strong base titration at the equivalence point.
Example: Titration of 25.00 mL of 0.100 M HCl with 0.100 M NaOH. At the equivalence point, the pH is 7.00.
2. Strong Acid - Weak Base Titration
At the equivalence point, the strong acid has completely neutralized the weak base, resulting in a solution containing the conjugate acid of the weak base. The conjugate acid will undergo hydrolysis, increasing the hydrogen ion concentration and lowering the pH.
Steps for Calculation:
-
Determine the moles of the weak base: Moles = Molarity × Volume (in Liters)
-
Determine the moles of the conjugate acid: At the equivalence point, moles of conjugate acid = moles of weak base initially present.
-
Calculate the concentration of the conjugate acid: Concentration = Moles / Total Volume (in Liters) Remember to add the volumes of acid and base used.
-
Use the Ka expression to find the hydronium ion concentration: The Ka of the conjugate acid is related to the Kb of the weak base by the equation Ka * Kb = Kw (where Kw is the ion product of water, 1.0 x 10^-14 at 25°C).
-
Calculate the pH: pH = -log[H3O+]
Example: Titration of 25.00 mL of 0.100 M NH₃ (Kb = 1.8 x 10⁻⁵) with 0.100 M HCl.
- Moles of NH₃ = 0.100 M * 0.025 L = 0.0025 moles
- Moles of NH₄⁺ (conjugate acid) = 0.0025 moles
- Total volume = 50.00 mL = 0.050 L
- [NH₄⁺] = 0.0025 moles / 0.050 L = 0.050 M
- Ka = Kw / Kb = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰
- Use the ICE table (Initial, Change, Equilibrium) to calculate [H3O+]:
- NH₄⁺ + H₂O ⇌ H₃O⁺ + NH₃
- Initial: 0.050 M, 0, 0
- Change: -x, +x, +x
- Equilibrium: 0.050-x, x, x
- Ka = x² / (0.050 - x) ≈ x² / 0.050 (since x is usually small compared to 0.050)
- x = [H3O+] = √(Ka * 0.050) = √(5.6 x 10⁻¹⁰ * 0.050) ≈ 5.3 x 10⁻⁶ M
- pH = -log(5.3 x 10⁻⁶) ≈ 5.28
3. Weak Acid - Strong Base Titration
Similar to the previous case, at the equivalence point, the strong base has completely neutralized the weak acid, leaving behind the conjugate base. This conjugate base undergoes hydrolysis, increasing the hydroxide ion concentration and raising the pH above 7.
Steps for Calculation:
-
Determine the moles of weak acid: Moles = Molarity × Volume (in Liters)
-
Determine the moles of the conjugate base: At the equivalence point, moles of conjugate base = moles of weak acid initially present.
-
Calculate the concentration of the conjugate base: Concentration = Moles / Total Volume (in Liters)
-
Use the Kb expression to find the hydroxide ion concentration: Kb = Kw / Ka.
-
Calculate the pOH: pOH = -log[OH⁻]
-
Calculate the pH: pH = 14 - pOH
Example: Titration of 25.00 mL of 0.100 M CH₃COOH (Ka = 1.8 x 10⁻⁵) with 0.100 M NaOH.
- Moles of CH₃COOH = 0.100 M * 0.025 L = 0.0025 moles
- Moles of CH₃COO⁻ (conjugate base) = 0.0025 moles
- Total volume = 50.00 mL = 0.050 L
- [CH₃COO⁻] = 0.0025 moles / 0.050 L = 0.050 M
- Kb = Kw / Ka = (1.0 x 10⁻¹⁴) / (1.8 x 10⁻⁵) = 5.6 x 10⁻¹⁰
- Use the ICE table to calculate [OH⁻]:
- CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻
- ... (similar steps as in the previous example)
- [OH⁻] ≈ 5.3 x 10⁻⁶ M
- pOH = -log(5.3 x 10⁻⁶) ≈ 5.28
- pH = 14 - 5.28 ≈ 8.72
4. Weak Acid - Weak Base Titration
This scenario is the most complex. The pH at the equivalence point depends on the relative strengths of the weak acid and weak base. There's no simple shortcut; you need to consider the equilibrium of both the conjugate acid and conjugate base and solve the resulting system of equations, often requiring iterative methods or numerical solvers. The calculations are significantly more involved and often require the use of more advanced equilibrium calculations. This typically involves the Henderson-Hasselbalch equation and iterative solutions due to the simultaneous presence of the conjugate acid and conjugate base and their influence on each other.
Importance of Accurate pH Calculation at the Equivalence Point
Accurate determination of the pH at the equivalence point is critical for several reasons:
-
Indicator Selection: The pH at the equivalence point dictates the choice of a suitable indicator for the titration. The indicator's color change range must encompass the equivalence point for accurate results.
-
Titration Curve Analysis: The equivalence point is a key feature of the titration curve. Understanding its pH helps analyze the titration data and extract valuable information about the analyte.
-
Understanding Reaction Stoichiometry: The pH at the equivalence point reinforces the stoichiometric relationship between the acid and base in the reaction.
Conclusion
Calculating the pH at the equivalence point requires understanding the acid-base chemistry involved. While strong acid-strong base titrations are straightforward, titrations involving weak acids or weak bases require careful consideration of hydrolysis and equilibrium calculations. This guide has provided a comprehensive overview of the different scenarios and the necessary calculations. Mastering these calculations is crucial for accurate analysis and interpretation of titration data. Remember to always double-check your calculations and consider using numerical solvers for more complex scenarios involving weak acid-weak base titrations.
Latest Posts
Latest Posts
-
List Three Physical Properties Of Water
Mar 26, 2025
-
When A Substance In A Reaction Is Oxidized It
Mar 26, 2025
-
What Happens To Electrons In Metallic Bonding
Mar 26, 2025
-
Label The Types Of Intercellular Junctions
Mar 26, 2025
-
Is Soil Renewable Or Nonrenewable Resource
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
