How To Determine Shape Of A Molecule
Muz Play
Mar 15, 2025 · 6 min read
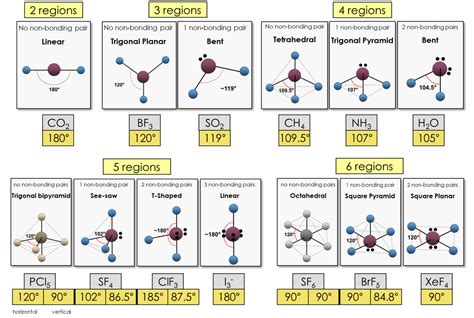
Table of Contents
How to Determine the Shape of a Molecule: A Comprehensive Guide
Determining the shape of a molecule is crucial in chemistry, as it dictates many of its physical and chemical properties. From reactivity to boiling point, the three-dimensional arrangement of atoms within a molecule significantly influences its behavior. This comprehensive guide will explore various methods and concepts used to predict and understand molecular geometry.
Understanding the Basics: Valence Shell Electron Pair Repulsion (VSEPR) Theory
The cornerstone of molecular shape prediction is the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory posits that the electron pairs surrounding a central atom will arrange themselves to minimize repulsion, thus determining the overall shape of the molecule. Electron pairs can be bonding pairs (shared between atoms) or lone pairs (unshared electrons). The key to understanding VSEPR lies in identifying these pairs and understanding how they interact.
Steps to Applying VSEPR Theory:
-
Draw the Lewis Structure: The first step involves drawing the Lewis structure of the molecule. This accurately represents the bonding and non-bonding electrons around each atom. This structure provides the foundation for determining the number of electron domains around the central atom.
-
Count Electron Domains: An electron domain refers to a region of electron density around the central atom. This includes both bonding pairs and lone pairs. For instance, a single bond, a double bond, and a triple bond all count as one electron domain each. A lone pair also counts as one electron domain.
-
Determine the Electron Domain Geometry: The number of electron domains dictates the electron domain geometry. This geometry represents the arrangement of all electron pairs (bonding and lone pairs) around the central atom. Common electron domain geometries include:
- Linear: Two electron domains (180° bond angle)
- Trigonal Planar: Three electron domains (120° bond angle)
- Tetrahedral: Four electron domains (109.5° bond angle)
- Trigonal Bipyramidal: Five electron domains
- Octahedral: Six electron domains
-
Determine the Molecular Geometry: This is the actual shape of the molecule, considering only the positions of the atoms. Lone pairs influence the molecular geometry but are not included in the description of the shape. For example, a molecule with four electron domains (tetrahedral electron domain geometry) but two lone pairs will have a bent molecular geometry.
-
Consider Bond Angles and Dipole Moments: The bond angles predicted by VSEPR are idealized. The presence of lone pairs or different atoms can cause deviations from these ideal angles. Furthermore, understanding the polarity of bonds and the overall molecular dipole moment can provide additional insights into the molecule's behavior.
Examples Illustrating VSEPR Theory:
Let's analyze the shapes of a few molecules using VSEPR:
Methane (CH₄):
- Lewis Structure: Carbon is the central atom surrounded by four hydrogen atoms, each with a single bond.
- Electron Domains: Four electron domains (all bonding pairs).
- Electron Domain Geometry: Tetrahedral
- Molecular Geometry: Tetrahedral (all bonding pairs, so electron domain and molecular geometry are identical)
- Bond Angle: Approximately 109.5°
Water (H₂O):
- Lewis Structure: Oxygen is the central atom with two hydrogen atoms and two lone pairs.
- Electron Domains: Four electron domains (two bonding pairs and two lone pairs).
- Electron Domain Geometry: Tetrahedral
- Molecular Geometry: Bent (due to the presence of two lone pairs)
- Bond Angle: Less than 109.5° (lone pairs exert stronger repulsive forces, compressing the H-O-H angle)
Ammonia (NH₃):
- Lewis Structure: Nitrogen is the central atom with three hydrogen atoms and one lone pair.
- Electron Domains: Four electron domains (three bonding pairs and one lone pair).
- Electron Domain Geometry: Tetrahedral
- Molecular Geometry: Trigonal Pyramidal (due to the presence of one lone pair)
- Bond Angle: Less than 109.5°
Beyond VSEPR: More Advanced Techniques
While VSEPR is a powerful tool for predicting molecular shapes, it has limitations, particularly with more complex molecules. Several other techniques offer a more refined understanding:
Molecular Orbital Theory:
Molecular orbital (MO) theory provides a more sophisticated description of bonding. It considers the combination of atomic orbitals to form molecular orbitals, which can be bonding, antibonding, or non-bonding. The occupation of these orbitals influences the overall electron density distribution and, consequently, the molecular shape. MO theory is particularly useful for explaining the shapes of molecules with delocalized electrons, like benzene.
X-ray Diffraction:
Experimental techniques like X-ray diffraction provide definitive information about molecular structure. By analyzing the diffraction pattern of X-rays passing through a crystalline sample, scientists can determine the precise positions of atoms within the molecule. This method is highly accurate but requires crystalline samples.
Electron Diffraction:
Similar to X-ray diffraction, electron diffraction uses a beam of electrons to interact with the sample. This technique is particularly useful for gaseous or liquid samples that may not form crystals suitable for X-ray diffraction.
Neutron Diffraction:
Neutron diffraction utilizes a beam of neutrons to probe the structure of a molecule. It's especially effective for locating hydrogen atoms, which are difficult to detect using X-ray diffraction.
Computational Chemistry:
Advanced computational methods, using quantum mechanics principles, can accurately predict molecular geometries. Software packages employ various levels of theory, from simpler semi-empirical methods to highly accurate ab initio calculations. These computational methods are becoming increasingly powerful and accessible, offering detailed insights into molecular structure and properties.
Factors Affecting Molecular Shape:
Several factors beyond the number of electron domains influence the final molecular shape:
-
Hybridization: The concept of orbital hybridization, where atomic orbitals combine to form hybrid orbitals, plays a significant role in determining the geometry. The type of hybridization (sp, sp², sp³, etc.) dictates the arrangement of electron domains.
-
Steric Effects: The size and bulkiness of atoms or groups attached to the central atom can cause steric hindrance, leading to deviations from ideal bond angles predicted by VSEPR.
-
Resonance: Molecules with resonance structures exhibit delocalized electrons, leading to an averaging of bond lengths and angles, which can affect the overall shape.
-
Hydrogen Bonding: Hydrogen bonds, strong intermolecular forces, can influence the overall arrangement of molecules in a substance, even if they don't directly affect the intramolecular geometry.
Importance of Knowing Molecular Shape:
Understanding molecular shape is crucial for various reasons:
-
Predicting Reactivity: The shape of a molecule determines the accessibility of its reactive sites, influencing its reactivity with other molecules.
-
Determining Physical Properties: Molecular shape affects properties like melting point, boiling point, solubility, and density.
-
Understanding Biological Function: The precise three-dimensional arrangement of atoms in biomolecules is essential for their function. Enzymes, for example, rely on specific shapes to interact with substrates.
-
Designing New Materials: Understanding molecular shape allows scientists to design new materials with desired properties, such as strength, conductivity, or optical properties.
Conclusion:
Determining the shape of a molecule is a multifaceted endeavor involving both theoretical and experimental approaches. While VSEPR theory provides a valuable initial understanding, more advanced techniques such as MO theory and experimental methods like X-ray diffraction are crucial for a comprehensive analysis. The accurate prediction and understanding of molecular shape is fundamental to many areas of chemistry and beyond, paving the way for advancements in material science, biochemistry, and other related fields. By mastering the concepts discussed here, you can confidently tackle the complexities of molecular geometry and unlock a deeper understanding of the fascinating world of molecules.
Latest Posts
Latest Posts
-
What Is A Property Of An Element
Mar 15, 2025
-
Which State Of Matter Has No Definite Shape Or Volume
Mar 15, 2025
-
A Starting Material In A Chemical Reaction
Mar 15, 2025
-
A Solution That Contains The Maximum Amount Of Solute
Mar 15, 2025
-
Electric Field Around A Positive Charge
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How To Determine Shape Of A Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
