How To Determine The Coordination Number
Muz Play
Mar 23, 2025 · 6 min read
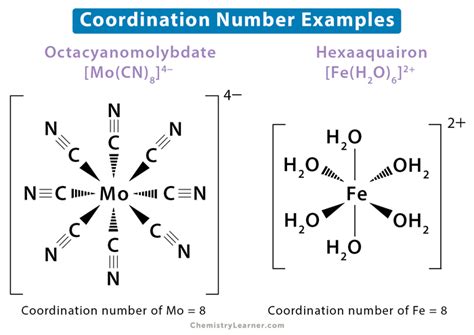
Table of Contents
How to Determine the Coordination Number: A Comprehensive Guide
Determining the coordination number of a central atom in a molecule or crystal structure is a fundamental concept in chemistry and materials science. The coordination number (CN) represents the number of atoms or ions directly bonded to a central atom. Understanding how to determine this number is crucial for predicting properties, understanding bonding, and interpreting crystal structures. This comprehensive guide will walk you through various methods and provide practical examples to solidify your understanding.
Understanding the Coordination Number
Before diving into the methods, let's clarify the concept. The coordination number isn't simply the number of atoms nearby; it specifically refers to the atoms directly bonded to the central atom. This direct bonding is usually defined by a significant overlap of electron orbitals or a strong electrostatic interaction. The distance between the central atom and its coordinating atoms also plays a significant role in determining the coordination number. Atoms at significantly longer distances are generally not included in the coordination sphere.
The coordination number significantly influences a compound's properties, including:
- Geometry: The CN dictates the spatial arrangement of the surrounding atoms, leading to different geometries like linear (CN=2), tetrahedral (CN=4), octahedral (CN=6), etc.
- Bonding: The nature of the bonds (ionic, covalent, metallic) and the electron distribution around the central atom are influenced by the CN.
- Stability: The stability of a compound can be related to the coordination environment of its central atom.
- Physical Properties: Melting point, boiling point, solubility, and other physical properties are often influenced by the coordination number.
Methods for Determining the Coordination Number
Several methods can be employed to determine the coordination number, depending on the available information and the complexity of the structure.
1. From Chemical Formulae (Simple Cases)
For simple ionic compounds, the coordination number can sometimes be deduced directly from the chemical formula. This is most reliable for compounds with relatively simple structures.
Example: In NaCl (sodium chloride), the formula suggests a 1:1 ratio of Na⁺ and Cl⁻ ions. In the crystal lattice, each Na⁺ ion is surrounded by six Cl⁻ ions, and each Cl⁻ ion is surrounded by six Na⁺ ions. Therefore, the coordination number of both Na⁺ and Cl⁻ in NaCl is 6.
Limitations: This method is limited to simple ionic compounds with predictable stoichiometry and crystal structures. It doesn't work for complex molecules or compounds with more intricate bonding.
2. Using Crystal Structures (X-ray Diffraction Data)
X-ray diffraction is a powerful technique to determine the three-dimensional arrangement of atoms in a crystal. Analyzing the diffraction pattern allows the precise determination of atomic positions and interatomic distances. By identifying the atoms directly surrounding the central atom within a specific distance threshold, one can determine the coordination number.
Process:
- Obtain X-ray diffraction data: This involves collecting diffraction data from a crystalline sample using an X-ray diffractometer.
- Structure refinement: The diffraction data is processed and analyzed using software to determine the atomic positions and bond lengths.
- Coordination sphere identification: Examine the refined structure and identify the atoms directly surrounding the central atom. The number of these atoms within a reasonable bonding distance constitutes the coordination number.
Advantages: This method provides precise information and is applicable to a wide range of crystalline materials.
Limitations: Requires specialized equipment and expertise in crystallography. Analyzing complex structures can be computationally intensive.
3. Analyzing Molecular Structures (Spectroscopy and Computational Methods)
For molecular compounds, various spectroscopic techniques and computational methods can be employed to determine the coordination number.
-
NMR Spectroscopy: Nuclear Magnetic Resonance (NMR) spectroscopy can provide information about the bonding environment of the central atom. The chemical shifts and coupling patterns can help determine the number of atoms directly bonded to the central atom.
-
IR Spectroscopy: Infrared (IR) spectroscopy can be used to identify specific functional groups and bonds surrounding the central atom. This information can be used to infer the coordination number.
-
Computational Chemistry: Density Functional Theory (DFT) and other computational methods can be used to model the molecule and determine the bond lengths and angles. This information can be used to identify the atoms directly bonded to the central atom and determine the coordination number.
Advantages: Applicable to molecular compounds and provides detailed information about bonding.
Limitations: Requires specialized equipment and expertise. Computational methods can be computationally expensive and may require approximations.
4. Visual Inspection of Molecular Models
For simple molecules, it's often possible to determine the coordination number through visual inspection of molecular models (ball-and-stick or space-filling models). By identifying the atoms directly bonded to the central atom, one can directly count the coordination number.
Example: In methane (CH₄), the carbon atom is surrounded by four hydrogen atoms, each forming a covalent bond. The coordination number of carbon in methane is 4.
Limitations: This method is limited to simple molecules where the structure is readily apparent. It's not applicable to complex molecules or extended structures.
5. Considering Ligand Properties
The nature of the ligands (atoms or molecules surrounding the central atom) can influence the coordination number. Steric hindrance (the spatial arrangement of ligands) can limit the number of ligands that can coordinate to the central atom. The charge and size of the ligand also play roles.
Example: Transition metal complexes often have various coordination numbers depending on the ligand size and electronic properties. Small, strongly coordinating ligands may lead to higher coordination numbers than large, weakly coordinating ligands.
Limitations: This method relies on an understanding of ligand properties and their influence on coordination chemistry. It's not a standalone method but rather a consideration alongside other methods.
Advanced Considerations and Examples
The determination of coordination number can become more complex in certain scenarios:
-
Bridging Ligands: When a ligand is bonded to more than one central atom (bridging ligand), careful consideration is needed to avoid double-counting. Each bond to the central atom needs to be considered individually.
-
Polymeric Structures: In extended polymeric structures, coordination numbers can vary depending on the location of the central atom in the polymer chain or network.
-
Fluxional Molecules: Some molecules undergo rapid changes in their structure (fluxional molecules), making the determination of a static coordination number challenging. In these cases, an average coordination number might be more appropriate.
-
Distorted Geometries: Deviations from ideal geometries can make determining the coordination number ambiguous. For instance, a slightly distorted octahedron may still be considered to have a coordination number of 6.
Examples of Coordination Number Determination:
-
[Co(NH₃)₆]³⁺: In the hexamminecobalt(III) ion, the cobalt(III) ion is surrounded by six ammonia molecules, each donating a lone pair of electrons. The coordination number of Co³⁺ is 6.
-
[Cu(H₂O)₄]²⁺: In the tetraaquacopper(II) ion, the copper(II) ion is coordinated to four water molecules. The coordination number of Cu²⁺ is 4.
-
TiO₂ (Rutile Structure): In the rutile structure of titanium dioxide, each Ti⁴⁺ ion is surrounded by six O²⁻ ions, and each O²⁻ ion is surrounded by three Ti⁴⁺ ions. The coordination number of Ti⁴⁺ is 6, and the coordination number of O²⁻ is 3.
Conclusion
Determining the coordination number is a crucial step in understanding the structure, properties, and reactivity of various compounds. The appropriate method for determining the coordination number depends on the nature of the compound and the available data. A combination of techniques, such as crystallography, spectroscopy, and computational methods, often yields the most reliable and complete understanding of the coordination environment. By mastering these methods, researchers can deepen their understanding of the fundamental principles governing the structure and behavior of matter. Careful consideration of bridging ligands, polymeric structures, and distorted geometries is essential for accurate determination in complex cases.
Latest Posts
Latest Posts
-
What Is The Smallest Unit Of Living Matter
Mar 24, 2025
-
The End Product Of Glycolysis Is
Mar 24, 2025
-
How Do You Know If A Reaction Is Spontaneous
Mar 24, 2025
-
Bond Length Between Two Bonded Atoms Is
Mar 24, 2025
-
A Cuantos Grados Fahrenheit Se Congela El Agua
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How To Determine The Coordination Number . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
