Bond Length Between Two Bonded Atoms Is
Muz Play
Mar 24, 2025 · 6 min read
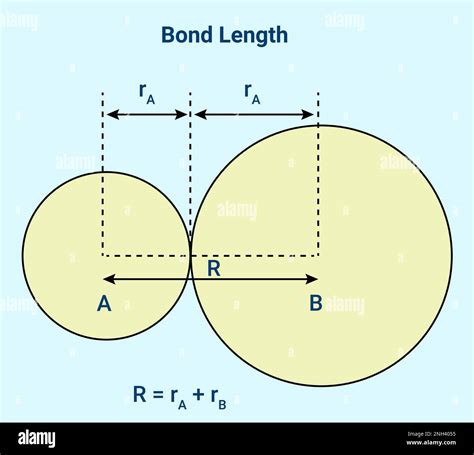
Table of Contents
Bond Length Between Two Bonded Atoms: A Deep Dive
The bond length between two bonded atoms is a fundamental concept in chemistry, representing the average distance between the nuclei of two atoms that are chemically bonded to each other. Understanding bond length is crucial for predicting and interpreting molecular properties, reactivity, and structure. This distance isn't static; it fluctuates due to vibrations, but the average distance provides invaluable insights into the nature of the chemical bond. This comprehensive article will explore the factors influencing bond length, the methods used to determine it, and its significance in various chemical contexts.
Factors Influencing Bond Length
Several factors intricately influence the bond length between two atoms. These factors interact in complex ways, making precise prediction challenging but allowing for a strong understanding of general trends:
1. Atomic Radii: The Foundation of Bond Length
The most significant factor is the atomic radii of the bonded atoms. Larger atoms generally lead to longer bond lengths because their nuclei are farther apart. This is intuitive: imagine trying to connect two large balloons versus two smaller ones; the distance between their centers will be greater for the larger balloons. Periodic trends in atomic radii, influenced by effective nuclear charge and shielding, directly translate to trends in bond length.
2. Bond Order: A Measure of Bond Strength
Bond order, representing the number of chemical bonds between two atoms, significantly impacts bond length. Higher bond orders generally lead to shorter bond lengths. Consider the carbon-carbon bond: a single bond (C-C) is longer than a double bond (C=C), which is, in turn, longer than a triple bond (C≡C). This is because a higher bond order implies stronger attraction between the atoms, pulling them closer together.
3. Hybridization: Shaping the Orbital Overlap
The hybridization of atomic orbitals involved in bond formation influences bond length. Different hybridization states lead to different orbital shapes and sizes, impacting the extent of orbital overlap. For instance, sp hybridized orbitals are smaller and more compact than sp³ hybridized orbitals, resulting in shorter bond lengths for sp hybridized bonds. This is particularly relevant in organic chemistry where comparing bond lengths in alkanes, alkenes, and alkynes showcases this effect.
4. Electronegativity: The Tug-of-War of Electrons
The electronegativity difference between bonded atoms affects bond length, although the effect is often subtle compared to bond order and atomic radii. When electronegativity difference is significant, the bond becomes more polar, with electrons shifting towards the more electronegative atom. This can lead to a slight shortening of the bond, as the increased electron density draws the atoms closer. However, this effect is often masked by other stronger influences.
5. Resonance: Electron Delocalization and Bond Length Equalization
In molecules exhibiting resonance, the electron density is delocalized across multiple bonds. This delocalization leads to an equalization of bond lengths. For example, in benzene, the carbon-carbon bonds are all of equal length, intermediate between the lengths of single and double bonds, showcasing the effect of electron delocalization on bond length.
6. Steric Effects: Spatial Constraints and Repulsions
Steric effects, arising from the spatial arrangement of atoms and groups, can influence bond length. Bulky substituents can cause steric hindrance, pushing bonded atoms slightly farther apart than expected based solely on the factors mentioned above. This effect is particularly pronounced in larger molecules with complex structures.
Methods for Determining Bond Length
Several experimental and computational methods are employed to determine bond lengths:
1. X-ray Crystallography: A Powerful Experimental Technique
X-ray crystallography is a highly precise experimental technique that provides accurate bond length measurements. By analyzing the diffraction pattern of X-rays scattered by a crystal, the positions of atoms in the crystal lattice can be determined, yielding precise bond lengths. This technique is widely used in structural chemistry and materials science.
2. Electron Diffraction: Studying Gases and Liquids
Electron diffraction is another experimental technique used to determine bond lengths, particularly for molecules in the gaseous or liquid phase. Similar to X-ray diffraction, this method analyzes the diffraction pattern of electrons scattered by the sample to determine atomic positions and thus bond lengths. It is particularly useful for molecules that are difficult to crystallize.
3. Microwave Spectroscopy: Measuring Rotational Transitions
Microwave spectroscopy measures the rotational transitions of molecules in the gas phase. The frequencies of these transitions are related to the moments of inertia of the molecule, which in turn depend on the bond lengths. This method is highly sensitive and provides accurate bond lengths for small, gas-phase molecules.
4. Computational Methods: Theoretical Predictions
Computational methods, such as density functional theory (DFT) and ab initio calculations, provide theoretical predictions of bond lengths. These methods solve the Schrödinger equation (or approximations thereof) to obtain the electronic structure of the molecule, from which bond lengths can be derived. While computational methods don't replace experimental measurements, they are valuable tools for predicting bond lengths in molecules that are difficult or impossible to study experimentally.
Significance of Bond Length in Chemistry
The significance of bond length extends across various fields of chemistry:
1. Structural Chemistry: Defining Molecular Geometry
Bond lengths are fundamental in structural chemistry, determining the three-dimensional geometry of molecules. The precise bond lengths and angles dictate the overall shape and symmetry of a molecule, influencing its physical and chemical properties.
2. Chemical Reactivity: Predicting Reaction Pathways
Bond lengths provide insights into the chemical reactivity of molecules. Shorter, stronger bonds are generally less reactive than longer, weaker bonds. Changes in bond length during a chemical reaction can indicate the progress of the reaction and provide insights into the reaction mechanism.
3. Spectroscopy: Interpreting Spectral Data
Bond lengths are directly related to spectroscopic data, particularly in vibrational spectroscopy (IR and Raman). The vibrational frequencies of molecules are influenced by the strength and length of bonds, allowing the interpretation of spectral data to yield information about bond lengths and molecular structure.
4. Materials Science: Designing Novel Materials
In materials science, understanding bond lengths is crucial for designing novel materials with specific properties. The strength, elasticity, and other properties of materials are directly related to the nature of the chemical bonds, including their lengths. Precise control over bond lengths is essential in the design of advanced materials.
5. Biochemistry and Drug Design: Understanding Biological Interactions
In biochemistry and drug design, bond lengths are vital for understanding biological interactions. The precise fit between molecules, like an enzyme and its substrate, is critically dependent on bond lengths and angles. This knowledge is crucial in drug discovery and development, allowing the design of molecules that specifically target biological systems.
Conclusion: A Fundamental Parameter with Broad Implications
The bond length between two bonded atoms is a deceptively simple yet incredibly significant parameter in chemistry. Its value is determined by a complex interplay of factors, including atomic radii, bond order, hybridization, electronegativity, resonance, and steric effects. Several experimental and computational techniques provide accurate measurements of bond lengths. Understanding bond length is crucial for interpreting molecular structure, predicting reactivity, designing novel materials, and understanding biological interactions, showcasing its fundamental role across diverse chemical disciplines. Continued research and development in both experimental and computational techniques will further refine our understanding of this crucial parameter and its impact on the chemical world.
Latest Posts
Latest Posts
-
State Of Matter With Definite Shape And Volume
Mar 26, 2025
-
Question Lexan Draw The Monomer Used To Make This Polymer
Mar 26, 2025
-
How Do You Calculate Index Numbers
Mar 26, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 26, 2025
-
What Is A Calorie In Chemistry
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Bond Length Between Two Bonded Atoms Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
