State Of Matter With Definite Shape And Volume
Muz Play
Mar 26, 2025 · 6 min read
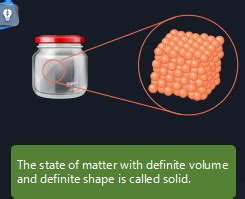
Table of Contents
The State of Matter with Definite Shape and Volume: A Deep Dive into Solids
The world around us is composed of matter, existing in various states or phases. These states are defined by the arrangement and movement of their constituent particles – atoms, ions, or molecules. While we commonly encounter solids, liquids, and gases, the distinctions are more nuanced than initially apparent. This article will delve into the fascinating world of solids, the state of matter characterized by a definite shape and volume. We'll explore the underlying principles that govern their behavior, the various types of solids, and their significance in our daily lives.
Understanding the Microscopic Structure of Solids
The defining characteristic of a solid is its rigidity. Unlike liquids and gases, solids resist changes in shape and volume. This resistance stems directly from the strong intermolecular forces between the constituent particles. In solids, these particles are tightly packed together in a highly ordered arrangement, often forming a repeating three-dimensional structure known as a crystal lattice.
The Role of Intermolecular Forces
The strength of the intermolecular forces dictates many of the solid's properties, including its melting point, hardness, and elasticity. These forces can be ionic bonds (like in table salt), covalent bonds (like in diamond), metallic bonds (like in iron), or weaker intermolecular forces like van der Waals forces (like in solid iodine). Stronger intermolecular forces generally lead to harder, higher-melting-point solids.
Crystal Structures: Order in the Solid State
The highly ordered arrangement of particles in a crystal lattice is responsible for the solid's definite shape. Different types of crystal structures exist, each with its own unique arrangement. Common crystal structures include:
- Cubic: Simple cubic, body-centered cubic, and face-centered cubic. These structures differ in how the particles are arranged within the cube.
- Tetragonal: Similar to cubic but with one axis longer or shorter than the others.
- Orthorhombic: Three unequal axes at right angles.
- Monoclinic: Three unequal axes, two at right angles, and one oblique.
- Triclinic: Three unequal axes, all at oblique angles.
- Hexagonal: A unique six-sided structure.
The specific crystal structure of a solid significantly influences its macroscopic properties. For example, the arrangement of carbon atoms in diamond (a giant covalent structure) makes it exceptionally hard, while the arrangement in graphite (layered structure) makes it soft and slippery.
Types of Solids: A Diverse Spectrum
Solids are not a monolithic group; they exhibit a wide range of properties and behaviors. We can categorize them based on their bonding characteristics and macroscopic properties.
Crystalline Solids: Order and Symmetry
Crystalline solids are characterized by their highly ordered, repeating structure. This order extends over large distances, creating a well-defined crystal lattice. The regularity of this structure is reflected in the physical properties of the crystal, often exhibiting distinct cleavage planes and anisotropic behavior (properties vary depending on the direction). Examples include quartz, diamond, and table salt.
Types of Crystalline Solids:
- Ionic solids: Held together by electrostatic forces between oppositely charged ions. They are usually hard, brittle, and have high melting points. Examples: NaCl, MgO.
- Covalent network solids: Atoms are covalently bonded throughout the entire structure forming a giant molecule. These are extremely hard and have very high melting points. Examples: Diamond, silicon dioxide (quartz).
- Metallic solids: Atoms are held together by metallic bonds, a sea of delocalized electrons. They are generally good conductors of electricity and heat, malleable, and ductile. Examples: Iron, copper, gold.
- Molecular solids: Individual molecules are held together by relatively weak intermolecular forces (van der Waals forces, hydrogen bonds). They tend to have lower melting points and are often soft. Examples: Ice, sugar, iodine.
Amorphous Solids: Disorder and Variability
In contrast to crystalline solids, amorphous solids lack a long-range ordered structure. Their atoms or molecules are arranged randomly, much like a liquid frozen in place. This lack of order leads to isotropic behavior (properties are the same in all directions). Examples include glass, rubber, and plastics. While they are considered solids due to their definite volume and shape, their structure is significantly different from crystalline materials. They often exhibit a gradual softening upon heating, rather than a sharp melting point.
Properties of Solids: A Closer Look
The properties of solids are intimately linked to their microscopic structure and the nature of the intermolecular forces. Here are some key properties:
Mechanical Properties:
- Hardness: A measure of a solid's resistance to scratching or indentation. Diamond is famously one of the hardest materials known.
- Strength: The ability of a solid to withstand stress without breaking.
- Ductility: The ability of a solid to be drawn into wires. Metals are generally ductile.
- Malleability: The ability of a solid to be hammered into thin sheets. Metals are also generally malleable.
- Elasticity: The ability of a solid to return to its original shape after deformation. Rubber is a highly elastic material.
Thermal Properties:
- Melting point: The temperature at which a solid transforms into a liquid. This is a characteristic property of a substance.
- Thermal conductivity: The ability of a solid to conduct heat. Metals are generally good thermal conductors.
- Specific heat capacity: The amount of heat required to raise the temperature of a solid by a certain amount.
Electrical Properties:
- Electrical conductivity: The ability of a solid to conduct electricity. Metals are good conductors, while many non-metals are insulators. Some materials exhibit semiconductivity, meaning their conductivity lies between conductors and insulators.
The Importance of Solids in Our World
Solids play a critical role in almost every aspect of our lives. From the buildings we live in to the electronic devices we use, solids form the foundation of our modern world. Their unique properties make them indispensable for a vast range of applications:
- Construction: Bricks, concrete, steel, and wood are just a few examples of solids used in construction.
- Electronics: Silicon, used in semiconductors, is the backbone of modern electronics.
- Medicine: Many pharmaceutical drugs are solids, and medical implants are often made from biocompatible solid materials.
- Transportation: The bodies of cars, trains, and airplanes are primarily made from solid materials.
- Packaging: Plastics and other solid materials are widely used for packaging various products.
Further Exploration: Advanced Concepts in Solid State Physics
The study of solids extends far beyond the basic concepts discussed here. Solid state physics is a vast and complex field encompassing many advanced topics, including:
- Band theory: Describes the behavior of electrons in solids and explains the difference between conductors, insulators, and semiconductors.
- Crystallography: The study of crystal structures and their properties.
- Defects in solids: Impurities and imperfections in the crystal lattice, which significantly affect the properties of the solid.
- Nanomaterials: Materials with dimensions on the nanoscale (1-100 nanometers), exhibiting unique properties due to their size.
Understanding the state of matter with definite shape and volume – the solid state – is fundamental to comprehending the physical world. From the intricate crystal structures of minerals to the advanced applications of nanomaterials, the realm of solids continues to fascinate and inspire scientists and engineers alike. The diverse properties and behaviors of solids make them crucial to our technology, infrastructure, and understanding of the universe. This deep dive has only scratched the surface of this vast and complex topic, highlighting the significance and intricate nature of this fundamental state of matter.
Latest Posts
Latest Posts
-
Type I And Type Ii Errors Examples
Mar 29, 2025
-
In Cellular Respiration Most Atp Molecules Are Produced By
Mar 29, 2025
-
What Is The Power Stroke In Muscle Contraction
Mar 29, 2025
-
What Is The Molecular Ion Peak
Mar 29, 2025
-
Genomics Can Be Used In Agriculture To
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about State Of Matter With Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
