How To Do Mole To Mass Conversions
Muz Play
Mar 23, 2025 · 6 min read
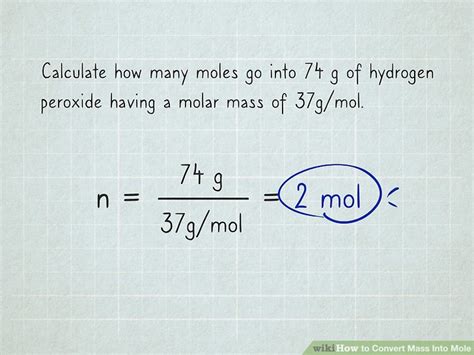
Table of Contents
How to Do Mole to Mass Conversions: A Comprehensive Guide
Mole to mass conversions are fundamental in chemistry, bridging the microscopic world of atoms and molecules to the macroscopic world of measurable quantities. Mastering this conversion is crucial for any aspiring chemist or anyone working with chemical calculations. This comprehensive guide will walk you through the process, explaining the underlying concepts and providing various examples to solidify your understanding. We'll delve into the importance of molar mass, explore different approaches to the conversion, and highlight common pitfalls to avoid.
Understanding the Fundamentals: Moles and Molar Mass
Before diving into the conversion itself, let's solidify our understanding of the key concepts: moles and molar mass.
What is a Mole?
The mole (mol) is the fundamental unit of measurement in chemistry for the amount of substance. It's essentially a counting unit, similar to a dozen (12 items) or a gross (144 items), but on a much larger scale. One mole contains Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of entities, which could be atoms, molecules, ions, or formula units. This enormous number reflects the incredibly small size of atoms and molecules.
What is Molar Mass?
Molar mass (M) represents the mass of one mole of a substance. It's expressed in grams per mole (g/mol). The molar mass of an element is numerically equal to its atomic weight (found on the periodic table) but with the unit g/mol. For compounds, the molar mass is the sum of the molar masses of all the atoms in the chemical formula.
Example: The molar mass of water (H₂O) is calculated as follows:
- Hydrogen (H): 1.01 g/mol x 2 atoms = 2.02 g/mol
- Oxygen (O): 16.00 g/mol x 1 atom = 16.00 g/mol
- Total Molar Mass (H₂O): 18.02 g/mol
The Conversion Process: Moles to Mass
The core of the mole-to-mass conversion lies in the relationship between moles, molar mass, and mass. This relationship can be expressed using the following formula:
Mass (g) = Moles (mol) x Molar Mass (g/mol)
This simple equation provides the framework for all mole-to-mass conversions. Let's break down how to apply this equation step-by-step.
Step-by-Step Guide:
-
Identify the given information: Determine the number of moles you are starting with. This will often be given directly in the problem or can be calculated from other given information.
-
Determine the molar mass: Calculate the molar mass of the substance involved in the conversion. Consult the periodic table to find the atomic masses of the elements in the compound. Remember to account for the number of each atom present in the chemical formula.
-
Apply the formula: Substitute the values for moles and molar mass into the equation: Mass (g) = Moles (mol) x Molar Mass (g/mol).
-
Calculate the mass: Perform the calculation to determine the mass in grams. Pay close attention to units to ensure they cancel correctly, leaving you with the desired unit of grams.
-
Check your work: Review your calculations to ensure accuracy and consistency in units.
Examples of Mole to Mass Conversions
Let's illustrate the conversion process with some practical examples:
Example 1: Converting Moles of a Monatomic Element to Mass
How many grams are in 2.5 moles of sodium (Na)?
- Given: 2.5 moles of Na
- Molar Mass: The molar mass of Na (from the periodic table) is approximately 22.99 g/mol.
- Calculation: Mass = Moles x Molar Mass = 2.5 mol x 22.99 g/mol = 57.48 g
- Answer: There are 57.48 grams in 2.5 moles of sodium.
Example 2: Converting Moles of a Compound to Mass
What is the mass in grams of 0.75 moles of carbon dioxide (CO₂)?
- Given: 0.75 moles of CO₂
- Molar Mass:
- Carbon (C): 12.01 g/mol
- Oxygen (O): 16.00 g/mol x 2 atoms = 32.00 g/mol
- Total Molar Mass (CO₂): 12.01 g/mol + 32.00 g/mol = 44.01 g/mol
- Calculation: Mass = Moles x Molar Mass = 0.75 mol x 44.01 g/mol = 33.01 g
- Answer: The mass of 0.75 moles of carbon dioxide is 33.01 grams.
Example 3: A More Complex Calculation
Calculate the mass of 1.2 x 10<sup>-3</sup> moles of sulfuric acid (H₂SO₄).
- Given: 1.2 x 10<sup>-3</sup> moles of H₂SO₄
- Molar Mass:
- Hydrogen (H): 1.01 g/mol x 2 atoms = 2.02 g/mol
- Sulfur (S): 32.07 g/mol
- Oxygen (O): 16.00 g/mol x 4 atoms = 64.00 g/mol
- Total Molar Mass (H₂SO₄): 2.02 g/mol + 32.07 g/mol + 64.00 g/mol = 98.09 g/mol
- Calculation: Mass = Moles x Molar Mass = 1.2 x 10<sup>-3</sup> mol x 98.09 g/mol = 0.1177 g
- Answer: The mass of 1.2 x 10<sup>-3</sup> moles of sulfuric acid is approximately 0.1177 grams.
Common Mistakes to Avoid
While the mole-to-mass conversion is straightforward, certain common mistakes can lead to inaccurate results. Here are some points to watch out for:
-
Incorrect Molar Mass Calculation: Double-check your calculation of the molar mass, paying close attention to the number of atoms of each element in the chemical formula.
-
Unit Errors: Always ensure your units cancel correctly throughout the calculation. Inconsistent or missing units can lead to significant errors.
-
Significant Figures: Pay attention to the significant figures in your given values and report your final answer with the correct number of significant figures.
-
Scientific Notation: When dealing with very small or very large numbers, use scientific notation to avoid errors and ensure clarity.
Beyond the Basics: Applications and Extensions
The mole-to-mass conversion is a fundamental skill with applications across various chemical concepts and calculations. It forms the basis for:
- Stoichiometry: Calculating reactant and product amounts in chemical reactions.
- Solution Chemistry: Determining the concentration of solutions in molarity (moles per liter).
- Titration Calculations: Analyzing the concentration of unknown solutions.
- Gas Laws: Relating the number of moles of a gas to its volume, pressure, and temperature.
Mastering this conversion provides a solid foundation for more advanced chemical calculations and a deeper understanding of chemical processes.
Conclusion
The mole-to-mass conversion is a cornerstone of chemical calculations. By understanding the underlying principles of moles and molar mass and following the steps outlined in this guide, you can confidently perform these conversions and apply them to a wide range of chemical problems. Remember to practice regularly, pay close attention to detail, and always double-check your work to ensure accuracy. With consistent practice, this essential skill will become second nature.
Latest Posts
Latest Posts
-
Environmental Factors That Influence Microbial Growth
Mar 25, 2025
-
Electrons In An Atoms Outermost Energy Shells Are Called
Mar 25, 2025
-
Which Kingdoms Contain Organisms That Are Prokaryotes
Mar 25, 2025
-
What Element Is Found In Proteins
Mar 25, 2025
-
2 Sample Z Test For Proportions
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How To Do Mole To Mass Conversions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
