How To Find Alpha On A Lineweaver Burk Plot
Muz Play
Mar 26, 2025 · 6 min read
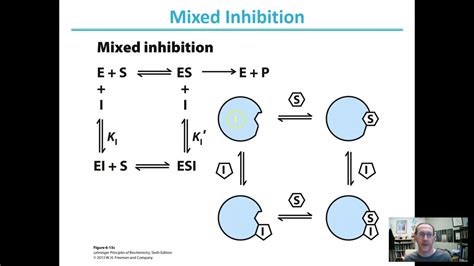
Table of Contents
How to Find Alpha on a Lineweaver-Burk Plot: A Comprehensive Guide
The Lineweaver-Burk plot, also known as a double reciprocal plot, is a graphical representation of the Michaelis-Menten equation. It's a valuable tool in enzymology for determining key kinetic parameters of enzyme-catalyzed reactions, including the Michaelis constant (Km) and the maximum reaction velocity (Vmax). However, its utility extends beyond these fundamental parameters. Understanding how to find alpha on a Lineweaver-Burk plot allows for the analysis of competitive, non-competitive, and uncompetitive enzyme inhibition. This comprehensive guide will delve into the intricacies of this process, providing a step-by-step explanation and clarifying common misconceptions.
Understanding the Lineweaver-Burk Plot and its Equation
The Lineweaver-Burk plot transforms the Michaelis-Menten equation, v = (Vmax[S])/(Km + [S]), into a linear form:
1/[v] = (Km/Vmax)(1/[S]) + 1/Vmax
Where:
- v represents the initial reaction velocity.
- Vmax represents the maximum reaction velocity.
- Km represents the Michaelis constant.
- [S] represents the substrate concentration.
Plotting 1/[v] against 1/[S] generates a straight line with a y-intercept of 1/Vmax and a slope of Km/Vmax. This linearization simplifies the determination of Km and Vmax from experimental data.
The Significance of Alpha (α) in Enzyme Inhibition
Alpha (α) is a crucial factor in analyzing enzyme inhibition kinetics using Lineweaver-Burk plots. It represents the factor by which the apparent Km or Vmax is altered in the presence of an inhibitor. The value of α directly reflects the type of inhibition:
-
Competitive Inhibition: In competitive inhibition, the inhibitor binds to the enzyme's active site, preventing substrate binding. This increases the apparent Km (Km'), but Vmax remains unchanged. Therefore, α = Km'/Km > 1. The Lineweaver-Burk plot shows parallel lines with different x-intercepts.
-
Non-competitive Inhibition: In non-competitive inhibition, the inhibitor binds to an allosteric site, altering the enzyme's conformation and reducing its catalytic efficiency. This decreases Vmax (Vmax'), but Km remains unchanged. Consequently, α = Vmax/Vmax' > 1. The Lineweaver-Burk plot shows lines intersecting at the y-axis.
-
Uncompetitive Inhibition: In uncompetitive inhibition, the inhibitor binds only to the enzyme-substrate complex. This decreases both Vmax and Km proportionally. Therefore, α = Km'/Km = Vmax/Vmax' > 1. The Lineweaver-Burk plot shows lines intersecting at the second or third quadrant.
-
Mixed Inhibition: Mixed inhibition involves a combination of competitive and non-competitive inhibition, resulting in changes to both Km and Vmax. α is used to describe the changes to both parameters.
Determining Alpha from a Lineweaver-Burk Plot: A Step-by-Step Approach
To determine α from a Lineweaver-Burk plot, follow these steps:
1. Conduct the Enzyme Assay: Perform enzyme assays with varying substrate concentrations, both in the absence and presence of the inhibitor. Ensure accurate measurement of initial reaction velocities (v) at each substrate concentration.
2. Calculate Reciprocals: Calculate the reciprocals of both the initial velocities (1/v) and the substrate concentrations (1/[S]) for all data points.
3. Plot the Data: Plot 1/v against 1/[S] on a graph. This will generate two lines: one for the uninhibited enzyme and one for the inhibited enzyme.
4. Determine Vmax and Km: Determine the Vmax and Km values for both the inhibited and uninhibited enzyme using the y-intercept (1/Vmax) and slope (Km/Vmax) of the lines. Remember that the y-intercept is 1/Vmax and the x-intercept is -1/Km.
5. Calculate Alpha (α): Depending on the type of inhibition, calculate α using the following formulas:
- Competitive Inhibition: α = Km'/Km (where Km' is the apparent Km in the presence of the inhibitor)
- Non-competitive Inhibition: α = Vmax/Vmax' (where Vmax' is the apparent Vmax in the presence of the inhibitor)
- Uncompetitive Inhibition: α = Km'/Km = Vmax/Vmax'
6. Interpret the Results: The value of α will indicate the type and strength of the inhibition. A larger α value indicates stronger inhibition.
Example: Calculating Alpha for Competitive Inhibition
Let's assume the following data from a Lineweaver-Burk plot for a competitive inhibitor:
- Uninhibited Enzyme: Km = 2 mM, Vmax = 10 µmol/min
- Inhibited Enzyme: Km' = 6 mM, Vmax' = 10 µmol/min
In this case, Vmax remains unchanged (as expected in competitive inhibition), while Km has increased. Therefore:
α = Km'/Km = 6 mM / 2 mM = 3
This indicates a significant competitive inhibition effect, where the apparent Km is three times higher in the presence of the inhibitor.
Common Mistakes and Pitfalls to Avoid
-
Inaccurate Data: Errors in experimental measurements will directly affect the accuracy of the Lineweaver-Burk plot and the calculated α value. Repeat experiments and ensure proper data handling are essential.
-
Ignoring Data Points: Discarding data points that don't fit the expected trend can lead to inaccurate calculations. All data points should be considered unless there is a clear reason to exclude them (e.g., outliers due to experimental error).
-
Non-Linearity: The Lineweaver-Burk plot assumes linearity. However, at very high or low substrate concentrations, the plot may deviate from linearity. This can lead to inaccurate estimations of Km and Vmax, impacting the α value calculation. Consider using alternative methods like the Eadie-Hofstee plot if significant non-linearity is observed.
-
Misinterpretation of Inhibition Types: It is crucial to understand the different types of enzyme inhibition before using the Lineweaver-Burk plot. Incorrect assignment of inhibition type leads to errors in interpreting the α value and drawing inaccurate conclusions.
Alternative Methods for Determining Kinetic Parameters
While the Lineweaver-Burk plot is widely used, it's important to acknowledge its limitations, particularly its sensitivity to errors in the measurement of low velocity values. Modern approaches often favor alternative methods such as:
-
Direct Linear Plot: This method directly plots the data without reciprocal transformations, making it less susceptible to error propagation.
-
Hanes-Woolf Plot: This plot ( [S]/v vs [S]) offers an alternative linearization of the Michaelis-Menten equation.
-
Eadie-Hofstee Plot: Another linear transformation (v vs v/[S]) that minimizes error propagation compared to the Lineweaver-Burk plot.
These alternative methods provide more robust estimations of Km and Vmax, leading to more reliable calculations of α. Choosing the appropriate method depends on the specific experimental context and data characteristics.
Conclusion: Mastering Alpha on the Lineweaver-Burk Plot
Determining alpha (α) on a Lineweaver-Burk plot is a powerful technique for analyzing enzyme inhibition kinetics. By understanding the underlying principles, following the appropriate steps, and being aware of potential pitfalls, researchers can effectively use this graphical method to characterize the type and strength of enzyme inhibition, providing valuable insights into enzyme mechanisms and drug design. Remember to carefully consider alternative plotting methods to minimize error and ensure the most reliable results. While the Lineweaver-Burk plot provides a valuable visualization, always critically assess the data and consider alternative methods to confirm your findings and gain a robust understanding of the enzyme kinetics.
Latest Posts
Latest Posts
-
What Is The Density Of Maple Syrup
Mar 29, 2025
-
What Is Stronger C C Bond Or C Cl Bond
Mar 29, 2025
-
Where Is The Bacterial Chromosome Located
Mar 29, 2025
-
Write The Chemical Formula For This Molecule
Mar 29, 2025
-
How To Calculate Velocity From Flow Rate
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How To Find Alpha On A Lineweaver Burk Plot . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
