How To Find Core And Valence Electrons
Muz Play
Mar 18, 2025 · 7 min read
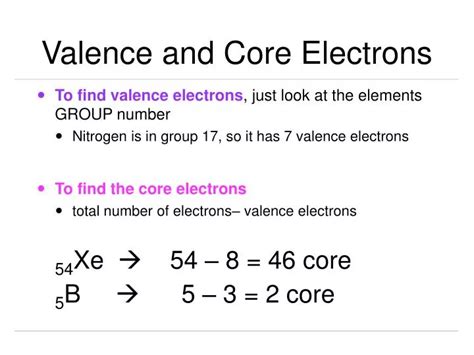
Table of Contents
How to Find Core and Valence Electrons: A Comprehensive Guide
Understanding the arrangement of electrons within an atom is fundamental to comprehending chemical bonding and reactivity. Electrons occupy specific energy levels and sublevels, and categorizing them into core and valence electrons provides a crucial framework for predicting an element's behavior. This comprehensive guide will explore the methods to determine the number of core and valence electrons, clarifying the concepts with numerous examples and explanations.
What are Core and Valence Electrons?
Before diving into the methods, let's define our key terms:
Core electrons are the electrons that occupy the inner energy levels of an atom. They are tightly bound to the nucleus and are generally not involved in chemical bonding. Their primary role is to shield the valence electrons from the full positive charge of the nucleus.
Valence electrons are the electrons located in the outermost energy level (valence shell) of an atom. These electrons are loosely held and are the primary participants in chemical reactions and the formation of chemical bonds. The number of valence electrons largely determines an element's chemical properties and reactivity.
Method 1: Using the Periodic Table
The periodic table is a powerful tool for quickly determining the number of valence electrons. Its structure directly reflects the electron configuration of elements.
Understanding the Periodic Table's Structure
The periodic table is arranged in periods (rows) and groups (columns). The period number corresponds to the principal quantum number (n) of the outermost electron shell. The group number (for groups 1-18) generally indicates the number of valence electrons.
- Groups 1 and 2 (Alkali and Alkaline Earth Metals): These elements have 1 and 2 valence electrons, respectively.
- Groups 13-18 (Boron Group to Noble Gases): For these groups, the number of valence electrons is equal to the group number minus 10. For example, Group 13 elements have 3 valence electrons (13 - 10 = 3).
- Transition Metals (Groups 3-12): Determining the valence electrons for transition metals is more complex, as they can involve electrons from multiple shells in bonding. It's often not straightforward and depends on the specific compound and its oxidation state.
- Lanthanides and Actinides (f-block elements): Similarly, determining valence electrons for these elements is more involved and usually necessitates a deeper understanding of their electronic configurations.
Examples using the Periodic Table:
- Oxygen (O): Oxygen is in Group 16, so it has 6 valence electrons (16 - 10 = 6).
- Sodium (Na): Sodium is in Group 1, so it has 1 valence electron.
- Chlorine (Cl): Chlorine is in Group 17, so it has 7 valence electrons (17 - 10 = 7).
- Aluminum (Al): Aluminum is in Group 13, so it has 3 valence electrons (13 - 10 = 3).
Finding Core Electrons using the Periodic Table: Once you've determined the number of valence electrons, finding the core electrons is straightforward. Simply subtract the number of valence electrons from the total number of electrons (equal to the atomic number).
- Oxygen (O, atomic number 8): 8 total electrons - 6 valence electrons = 2 core electrons.
- Sodium (Na, atomic number 11): 11 total electrons - 1 valence electron = 10 core electrons.
Method 2: Using Electron Configurations
Electron configuration describes the arrangement of electrons within an atom's energy levels and sublevels. This method provides a more precise and detailed approach to determining core and valence electrons.
Understanding Electron Configurations
Electron configurations are written using a notation that specifies the principal quantum number (n), the type of sublevel (s, p, d, f), and the number of electrons in each sublevel. For example, the electron configuration of oxygen (O) is 1s²2s²2p⁴.
- Principal Quantum Number (n): Represents the energy level (shell).
- Sublevels (s, p, d, f): Represent the subshells within each energy level.
- Superscripts: Indicate the number of electrons in each sublevel.
Identifying Core and Valence Electrons from Electron Configurations
- Valence electrons: These are the electrons in the outermost energy level (highest principal quantum number).
- Core electrons: These are all the electrons except those in the outermost energy level.
Examples using Electron Configurations:
- Oxygen (O): 1s²2s²2p⁴. The outermost energy level is n=2, containing 2s²2p⁴ = 6 electrons. Therefore, oxygen has 6 valence electrons. The core electrons are those in the n=1 level (1s² = 2 electrons).
- Sodium (Na): 1s²2s²2p⁶3s¹. The outermost energy level is n=3, containing 1 electron (3s¹). Sodium has 1 valence electron. The core electrons are in the n=1 and n=2 levels (1s²2s²2p⁶ = 10 electrons).
- Chlorine (Cl): 1s²2s²2p⁶3s²3p⁵. The outermost energy level is n=3, containing 3s²3p⁵ = 7 electrons. Chlorine has 7 valence electrons. The core electrons are in n=1 and n=2 levels (1s²2s²2p⁶ = 10 electrons).
- Iron (Fe): [Ar] 3d⁶4s². The outermost electrons are in the n=4 shell (4s²), so it has 2 valence electrons (simplified). The core electrons include those represented by [Ar] (18) plus the 3d⁶ electrons (6), totaling 24 core electrons. Note that transition metal valence electron determination is more nuanced.
Method 3: Using the Aufbau Principle and Hund's Rule
The Aufbau principle states that electrons fill atomic orbitals in order of increasing energy. Hund's rule adds that electrons will singly occupy orbitals within a subshell before pairing up. These principles, combined with the periodic table and electron configuration, provide another way to identify core and valence electrons.
Applying Aufbau and Hund's Rule
Using the Aufbau principle, we can systematically fill orbitals according to their energy levels. Hund's rule ensures that we distribute electrons evenly within a subshell before pairing them up. This process allows us to determine the complete electron configuration, from which we can identify core and valence electrons as described in Method 2.
Exceptions and Complications
While the methods described above work well for most elements, some exceptions exist, particularly among transition metals and lanthanides/actinides. Their electron configurations and valence electron counts can deviate from the simple rules outlined above due to complex electron-electron interactions and relativistic effects.
For example, chromium (Cr) has an electron configuration of [Ar] 3d⁵4s¹, not the expected [Ar] 3d⁴4s², due to the stability gained by having a half-filled d subshell. Similarly, copper (Cu) has [Ar] 3d¹⁰4s¹, favoring a completely filled d subshell. These exceptions highlight the complexities of electron behavior in larger atoms.
Importance of Understanding Core and Valence Electrons
The ability to determine core and valence electrons is crucial for a variety of reasons:
- Predicting Chemical Bonding: The number of valence electrons determines how an element will bond with other elements. Elements strive to achieve a stable electron configuration (often an octet, eight valence electrons), leading to the formation of ionic or covalent bonds.
- Understanding Reactivity: Elements with fewer or more valence electrons are generally more reactive than those with a full valence shell (like noble gases).
- Explaining Periodic Trends: Trends in ionization energy, electronegativity, and atomic radius are closely linked to the number of valence electrons and their shielding by core electrons.
- Basis for Chemical Calculations: Understanding electron configuration and the distinction between core and valence electrons is essential for various chemical calculations, including bond energy calculations and electron affinity determination.
Conclusion
Determining the number of core and valence electrons is a fundamental skill in chemistry. The periodic table provides a quick method for estimating valence electrons, while electron configurations offer a precise and detailed understanding of electron distribution within an atom. While exceptions exist, particularly among transition metals, a thorough understanding of these methods is essential for comprehending chemical bonding, reactivity, and numerous other chemical concepts. By mastering these techniques, you'll significantly enhance your ability to interpret and predict the behavior of elements and their compounds.
Latest Posts
Latest Posts
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How To Find Core And Valence Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
