How To Find Density Of A Gas
Muz Play
Mar 26, 2025 · 7 min read
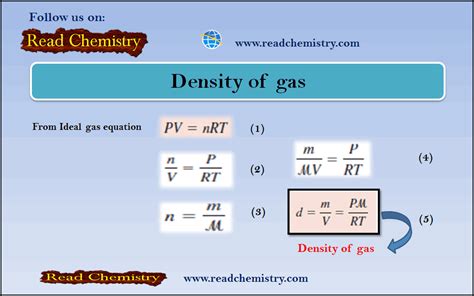
Table of Contents
How to Find the Density of a Gas: A Comprehensive Guide
Determining the density of a gas is a fundamental task in various scientific and engineering disciplines. From atmospheric science and chemical engineering to environmental monitoring and industrial process control, understanding gas density is crucial. Unlike solids and liquids, gases are highly compressible and their density is significantly affected by temperature and pressure. This comprehensive guide will explore various methods for determining gas density, detailing the underlying principles and providing practical considerations for accurate measurement.
Understanding Gas Density
Before diving into the methods, let's establish a clear understanding of what gas density is. Gas density (ρ) is defined as the mass (m) of a gas per unit volume (V):
ρ = m/V
Unlike solids and liquids, the density of a gas is not a constant value. It's heavily dependent on two key factors:
1. Pressure (P):
According to Boyle's Law, at a constant temperature, the volume of a gas is inversely proportional to its pressure. Higher pressure means the gas molecules are compressed into a smaller volume, leading to a higher density.
2. Temperature (T):
According to Charles's Law, at a constant pressure, the volume of a gas is directly proportional to its absolute temperature. Higher temperature means the gas molecules move faster and occupy a larger volume, resulting in a lower density.
Methods for Determining Gas Density
Several methods exist for determining the density of a gas, each with its own advantages and limitations. The choice of method depends on factors like the accuracy required, the available equipment, and the properties of the gas itself.
1. Using the Ideal Gas Law
The Ideal Gas Law provides a theoretical approach to calculating gas density. This law is a good approximation for many gases under standard conditions, but it becomes less accurate at high pressures and low temperatures where intermolecular forces become significant. The Ideal Gas Law is expressed as:
PV = nRT
Where:
- P is the pressure of the gas
- V is the volume of the gas
- n is the number of moles of the gas
- R is the ideal gas constant (8.314 J/mol·K)
- T is the absolute temperature of the gas (in Kelvin)
To find density using the Ideal Gas Law, we need to rearrange the equation to solve for density (ρ = m/V). Since the number of moles (n) is equal to the mass (m) divided by the molar mass (M), we can substitute this into the Ideal Gas Law:
PV = (m/M)RT
Rearranging to solve for density (ρ = m/V):
ρ = (PM)/(RT)
This equation allows us to calculate the density of a gas if we know its pressure, temperature, and molar mass. Remember to use consistent units throughout the calculation.
Example: Calculate the density of oxygen (O2) at 25°C and 1 atm pressure. The molar mass of O2 is 32 g/mol.
First, convert Celsius to Kelvin: 25°C + 273.15 = 298.15 K
Then, substitute the values into the equation:
ρ = (1 atm * 32 g/mol) / (0.0821 L·atm/mol·K * 298.15 K) ≈ 1.31 g/L
2. Using a Gas Density Meter
Gas density meters provide a direct measurement of gas density. These instruments employ various principles, including:
-
Buoyancy-based methods: These meters measure the buoyant force exerted on a known volume by the gas. The difference in buoyant force between the gas and a reference gas (often air) is directly related to the gas density.
-
Oscillating-element methods: These meters utilize a vibrating element whose resonant frequency is sensitive to the density of the surrounding gas. The change in frequency provides a measure of gas density.
-
Coriolis-effect based methods: These instruments utilize the Coriolis effect to measure mass flow and volume flow, enabling accurate density determination.
Gas density meters offer high accuracy and are suitable for real-time monitoring of gas density in various industrial processes. They often incorporate temperature and pressure sensors for compensation and provide digital readouts of gas density.
3. Using the Pressure-Volume Relationship (Experimental Method)
This method involves measuring the mass and volume of a gas sample under controlled conditions. A known volume container is evacuated, and then the gas is introduced at a known temperature and pressure. The increase in mass is directly related to the mass of the gas, and we can directly calculate the density using ρ = m/V.
Procedure:
-
Weigh the empty container: Accurately weigh the container (e.g., a flask or cylinder) that will hold the gas sample. Note down this mass (m_container).
-
Fill the container with the gas: Carefully fill the container with the gas at a known temperature and pressure using a calibrated pressure gauge and thermometer.
-
Weigh the filled container: Accurately weigh the container filled with the gas (m_filled).
-
Calculate the mass of the gas: Subtract the mass of the empty container from the mass of the filled container to obtain the mass of the gas (m_gas = m_filled - m_container).
-
Determine the volume of the gas: Measure or calculate the volume of the container (V).
-
Calculate the density: Divide the mass of the gas by its volume to obtain the density (ρ = m_gas/V).
This method provides a relatively straightforward way to determine gas density, particularly for gases that are easily handled and contained. Accuracy depends heavily on the accuracy of the weighing and volume measurements.
4. Using the Specific Gravity Method (Relative Density)
Specific gravity, also known as relative density, is the ratio of the density of a gas to the density of a reference gas, usually air or another gas with a known density at the same temperature and pressure. This method is convenient when an absolute density measurement is not required. The specific gravity is often determined by specialized instruments designed to compare the density of the unknown gas to a standard.
Specific Gravity = Density of Gas / Density of Reference Gas
The specific gravity can be used to estimate the density of the gas if the density of the reference gas is known.
Factors Affecting Accuracy
Several factors can impact the accuracy of gas density measurements:
-
Temperature fluctuations: Maintaining a stable temperature is crucial, as even small changes can significantly affect gas density. Use accurate temperature sensors and ensure good thermal insulation of the measurement apparatus.
-
Pressure variations: Similar to temperature, accurate pressure measurement is vital. Use calibrated pressure gauges and ensure that pressure is stable during measurements.
-
Gas purity: Impurities in the gas sample can affect its density. Ensure the gas sample is pure or know the composition of impurities and account for them in the calculations.
-
Calibration of instruments: Regular calibration of instruments (e.g., gas density meters, pressure gauges, and thermometers) is crucial to ensure accurate readings.
-
Measurement errors: Account for potential errors in weighing, volume measurement, temperature and pressure readings, and follow proper measurement techniques to minimize errors.
Applications of Gas Density Measurement
The determination of gas density finds extensive application across numerous fields:
-
Environmental Monitoring: Measuring the density of atmospheric gases (like CO2, methane, etc.) helps in understanding climate change, pollution levels, and greenhouse gas emissions.
-
Industrial Process Control: Monitoring gas density in chemical processes, pipelines, and refineries ensures optimal operational efficiency and safety.
-
Aerospace Engineering: Gas density is crucial in aerodynamic calculations and designing aircraft and spacecraft.
-
HVAC Systems: Gas density measurements optimize heating, ventilation, and air conditioning systems for energy efficiency and comfort.
-
Medical Applications: Gas density plays a role in respiratory gas analysis and anesthesia monitoring.
-
Food Industry: Measuring gas density in packaging helps to maintain food quality and shelf life.
-
Combustion Analysis: Accurate determination of the density of reactant and product gases is essential in combustion processes.
Conclusion
Determining the density of a gas requires careful consideration of various factors. Several methods, ranging from using the ideal gas law for theoretical calculations to employing sophisticated gas density meters for direct measurement, are available. The best method depends on the application, required accuracy, and available resources. By understanding the underlying principles and taking appropriate precautions, accurate gas density measurements can be obtained, providing crucial data for a wide range of scientific and engineering applications. Remember always to prioritize safety and follow proper laboratory procedures when handling gases.
Latest Posts
Latest Posts
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
-
What Are The Vertical Columns Called On A Periodic Table
Mar 29, 2025
-
Where Does Fermentation Take Place In A Cell
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How To Find Density Of A Gas . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
