How To Find Ph At Equivalence Point
Muz Play
Mar 16, 2025 · 6 min read
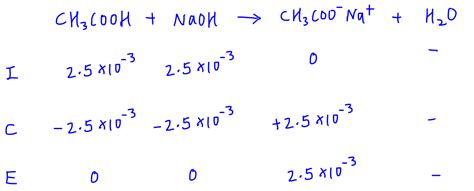
Table of Contents
How to Find pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial for understanding the reaction's stoichiometry and selecting appropriate indicators. This comprehensive guide delves into various methods for calculating and estimating this important value, catering to different titration scenarios and levels of chemical understanding. We'll explore both strong acid-strong base, weak acid-strong base, and weak base-strong acid titrations, providing practical examples and clarifying common misconceptions.
Understanding the Equivalence Point
The equivalence point in a titration is the point at which the moles of titrant added are stoichiometrically equal to the moles of analyte present. This signifies the complete neutralization of the analyte. It's important to differentiate this from the endpoint, which is the point at which the indicator changes color, signifying the approximate equivalence point. Ideally, the endpoint and equivalence point should be as close as possible.
The pH at the equivalence point isn't always 7. This is a common misconception. The pH depends heavily on the nature of the acid and base involved.
Calculating pH at the Equivalence Point: Different Scenarios
The methods for calculating the pH at the equivalence point vary depending on the strength of the acid and base involved. Let's examine the most common scenarios:
1. Strong Acid-Strong Base Titration
This is the simplest case. Since both the acid and base completely dissociate, the pH at the equivalence point is 7. This is because the resulting solution contains only water and a salt, which doesn't significantly affect the pH.
Example: Titrating 25.0 mL of 0.100 M HCl (strong acid) with 0.100 M NaOH (strong base).
At the equivalence point, the moles of HCl = moles of NaOH. The volume of NaOH required is 25.0 mL. The resulting solution is NaCl and water, resulting in a neutral pH of 7.
2. Weak Acid-Strong Base Titration
This is more complex. At the equivalence point, the weak acid is completely neutralized, forming a conjugate base. This conjugate base will undergo hydrolysis, producing hydroxide ions (OH⁻) and raising the pH above 7.
Calculating the pH:
-
Determine the concentration of the conjugate base: At the equivalence point, the moles of conjugate base are equal to the initial moles of weak acid. Calculate the concentration by considering the total volume of the solution.
-
Use the Kb expression: The Kb of the conjugate base is related to the Ka of the weak acid by the following equation: Kb = Kw / Ka, where Kw is the ion product constant for water (1.0 x 10⁻¹⁴ at 25°C).
-
Set up an ICE table: Create an ICE (Initial, Change, Equilibrium) table to determine the hydroxide ion concentration [OH⁻] at equilibrium.
-
Calculate pOH: pOH = -log[OH⁻]
-
Calculate pH: pH = 14 - pOH
Example: Titrating 25.0 mL of 0.100 M acetic acid (Ka = 1.8 x 10⁻⁵) with 0.100 M NaOH.
At the equivalence point, all acetic acid is converted to acetate ions. The total volume is 50.0 mL. The concentration of acetate is 0.050 M. Using the Kb expression and an ICE table, we can calculate [OH⁻] and subsequently pH, which will be greater than 7.
Simplified Calculation (Approximation): For weak acids with Ka < 10⁻⁴, a simplified calculation can often be used:
pH = 7 + ½(pKa + logC) where C is the concentration of the conjugate base. This is an approximation and gives a reasonable estimation.
3. Weak Base-Strong Acid Titration
This is analogous to the weak acid-strong base titration, but in reverse. At the equivalence point, the weak base is completely neutralized, forming a conjugate acid. This conjugate acid will undergo hydrolysis, producing hydronium ions (H₃O⁺) and lowering the pH below 7.
Calculating the pH: The process is similar to the weak acid-strong base titration but uses the Ka of the conjugate acid (Ka = Kw / Kb) and an ICE table to determine the hydronium ion concentration [H₃O⁺]. Then calculate pH = -log[H₃O⁺]. Again a simplified approximation can be applied under certain circumstances.
Simplified Calculation (Approximation): For weak bases with Kb < 10⁻⁴, a simplified calculation can often be used:
pH = 7 - ½(pKb + logC) where C is the concentration of the conjugate acid.
Dealing with Polyprotic Acids and Bases
Polyprotic acids and bases have multiple equivalence points, each corresponding to the neutralization of a proton (acid) or hydroxide ion (base). The calculations become more complex, often requiring iterative methods or software solutions to solve multiple equilibrium equations. Each equivalence point will have a different pH.
Estimating pH at the Equivalence Point using Indicators
Titration indicators are weak acids or bases that change color within a specific pH range. The choice of indicator depends on the expected pH at the equivalence point. The ideal indicator changes color close to the equivalence point, minimizing error.
For example:
- Strong acid-strong base titrations: Phenolphthalein (pH range 8.3-10.0) or bromothymol blue (pH range 6.0-7.6) are often used.
- Weak acid-strong base titrations: Phenolphthalein is commonly used, although the equivalence point pH might fall outside the ideal range; careful observation is necessary.
- Weak base-strong acid titrations: Methyl orange (pH range 3.1-4.4) or methyl red (pH range 4.4-6.2) are often used.
The color change of the indicator marks the endpoint, which is an approximation of the equivalence point.
Factors Affecting pH at the Equivalence Point
Several factors can influence the pH at the equivalence point:
- Temperature: The Kw of water changes with temperature, affecting the pH calculations, especially for weak acid-strong base and weak base-strong acid titrations.
- Ionic Strength: The presence of ions in the solution can affect the activity of the ions involved, slightly altering the pH.
- Activity Coefficients: These correct for deviations from ideal behavior in concentrated solutions. They are often omitted in introductory calculations but become important for precise work.
Advanced Techniques and Software
For more complex titrations or situations requiring high precision, advanced techniques and software are employed. These include potentiometric titrations (using a pH meter to monitor the pH continuously) and sophisticated equilibrium calculations using computational chemistry software.
Conclusion
Finding the pH at the equivalence point is a fundamental concept in acid-base chemistry. While strong acid-strong base titrations are straightforward, calculations for weak acid-strong base and weak base-strong acid titrations require careful consideration of equilibrium and hydrolysis. This guide provides a comprehensive overview of the methodologies, highlighting both exact and approximate calculation techniques, alongside the practical considerations of indicator selection and potential sources of error. Remember to always consider the specific nature of the acid and base involved when selecting calculation methods and indicators to ensure accurate results. Understanding these principles is essential for anyone working with acid-base titrations, whether in academic research or industrial applications.
Latest Posts
Latest Posts
-
Determine The Reactions At The Supports
Mar 16, 2025
-
A Mixture In Which The Composition Is Uniform Throughout
Mar 16, 2025
-
How To Break The Nitrogen Off An Imine Mechanism
Mar 16, 2025
-
Changes Color At The Endpoint Of A Titration
Mar 16, 2025
-
An Element That Conducts Heat And Electricity Poorly
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about How To Find Ph At Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
