How To Find Ph At The Equivalence Point
Muz Play
Mar 22, 2025 · 6 min read
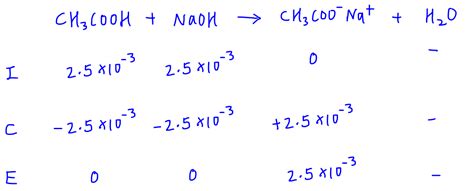
Table of Contents
How to Find pH at the Equivalence Point: A Comprehensive Guide
Determining the pH at the equivalence point of a titration is crucial in analytical chemistry, providing valuable insights into the strength of acids and bases. This comprehensive guide will delve into various methods for calculating and understanding the pH at this critical point, equipping you with the knowledge to tackle diverse titration scenarios.
Understanding the Equivalence Point
The equivalence point in a titration represents the point at which the moles of titrant added are stoichiometrically equivalent to the moles of analyte present. This doesn't automatically mean the pH is 7; that's only true for strong acid-strong base titrations. The pH at the equivalence point depends heavily on the nature of the acid and base involved (strong or weak).
Strong Acid-Strong Base Titration
In a strong acid-strong base titration, the equivalence point occurs at pH 7. This is because the reaction between a strong acid (completely dissociates) and a strong base (completely dissociates) produces a neutral salt and water. The resulting solution has equal concentrations of H⁺ and OH⁻ ions, resulting in a neutral pH. Calculating the pH is relatively straightforward at this point; the solution essentially contains only the salt, and if you know the concentration of the salt formed, you can calculate the pH.
Strong Acid-Weak Base Titration
Here, the equivalence point pH will be less than 7 (acidic). The weak base's conjugate acid is formed at the equivalence point, which will hydrolyze (react with water) to produce hydronium ions (H₃O⁺), lowering the pH. Calculating the pH requires considering the equilibrium of the conjugate acid's hydrolysis. You'll need the Ka (acid dissociation constant) of the conjugate acid to solve this. The pH can be calculated using the following steps:
- Determine the concentration of the conjugate acid: This requires knowing the initial moles of the weak base and the volume of strong acid added at the equivalence point.
- Set up an ICE table (Initial, Change, Equilibrium): This helps track the changes in concentrations of the conjugate acid, its conjugate base, and hydronium ions during the hydrolysis.
- Use the Ka expression: The Ka expression relates the concentrations of the conjugate acid, its conjugate base, and hydronium ions at equilibrium. Solve for [H₃O⁺].
- Calculate the pH: pH = -log₁₀[H₃O⁺]
Example: Titrating a solution of ammonia (NH₃) with hydrochloric acid (HCl). At the equivalence point, ammonium ion (NH₄⁺) is formed, which then undergoes hydrolysis. The Ka of NH₄⁺ will be used in this calculation.
Weak Acid-Strong Base Titration
In this case, the equivalence point pH will be greater than 7 (basic). The conjugate base of the weak acid is formed, which hydrolyzes to produce hydroxide ions (OH⁻), raising the pH. Similar to the strong acid-weak base titration, you will need the Kb (base dissociation constant) of the conjugate base to calculate the pH. The process involves:
- Determine the concentration of the conjugate base: This requires knowing the initial moles of the weak acid and the volume of strong base added at the equivalence point.
- Set up an ICE table: This tracks changes in the concentrations of the conjugate base, its conjugate acid, and hydroxide ions during hydrolysis.
- Use the Kb expression: The Kb expression relates the concentrations of the conjugate base, its conjugate acid, and hydroxide ions at equilibrium. Solve for [OH⁻].
- Calculate the pOH: pOH = -log₁₀[OH⁻]
- Calculate the pH: pH = 14 - pOH
Example: Titrating acetic acid (CH₃COOH) with sodium hydroxide (NaOH). At the equivalence point, acetate ion (CH₃COO⁻) is formed, which undergoes hydrolysis. The Kb of CH₃COO⁻ will be used for pH calculation.
Weak Acid-Weak Base Titration
These titrations are more complex. The pH at the equivalence point is determined by the relative strengths of the weak acid and weak base. A simple calculation using only Ka or Kb is insufficient. Instead, you'll need to consider the equilibrium of both the conjugate acid and conjugate base and use the appropriate equilibrium constants. Solving this often requires iterative methods or numerical techniques. The pH will be near 7 only if the acid and base have similar Ka and Kb values.
Finding the Equivalence Point
Before calculating the pH at the equivalence point, you must first determine the equivalence point itself. This is typically done experimentally using a pH meter or an indicator.
Using a pH Meter
A pH meter provides continuous pH readings during the titration. The equivalence point is identified by plotting the pH versus the volume of titrant added. The equivalence point is found at the steepest point of the curve (the inflection point). This method is highly accurate.
Using Indicators
Acid-base indicators are substances that change color over a specific pH range. The choice of indicator depends on the pH at the equivalence point. The indicator should change color around the equivalence point for a clear visual indication. While visually convenient, this method is less precise than using a pH meter.
Advanced Considerations
- Ionic Strength: The ionic strength of the solution can affect the activity of ions and thus the pH. In precise calculations, activity coefficients should be considered.
- Temperature: Equilibrium constants (Ka and Kb) are temperature-dependent. Calculations should use the appropriate constants for the given temperature.
- Polyprotic Acids and Bases: Titrations involving polyprotic acids or bases (those that can donate or accept more than one proton) have multiple equivalence points, each requiring individual pH calculations.
Practical Applications
Understanding pH at the equivalence point has numerous applications:
- Determining the concentration of unknown solutions: Titration is a fundamental technique for determining the concentration of unknown acids or bases.
- Acid-base equilibrium studies: It provides information about the relative strengths of acids and bases.
- Environmental monitoring: Determining pH is vital in monitoring water quality and soil acidity.
- Pharmaceutical analysis: Titration is used in the quality control of pharmaceutical products.
Conclusion
Determining the pH at the equivalence point of a titration is a crucial aspect of analytical chemistry. The approach varies significantly depending on the nature of the acid and base involved. While strong acid-strong base titrations have a straightforward pH of 7 at the equivalence point, weak acid-strong base, strong acid-weak base, and weak acid-weak base titrations require careful consideration of equilibrium constants and hydrolysis reactions. Accurate determination of the equivalence point itself, whether via a pH meter or indicator, is also crucial for precise pH calculations. By understanding these principles and employing appropriate techniques, one can accurately determine and interpret the pH at the equivalence point, providing valuable insights into chemical systems.
Latest Posts
Latest Posts
-
Does Sulfur Follow The Octet Rule
Mar 23, 2025
-
Temperature And Kinetic Energy Have A Relationship
Mar 23, 2025
-
Water Vascular System Of A Sea Star
Mar 23, 2025
-
Element Families Of The Periodic Table
Mar 23, 2025
-
Onto Vs One To One Linear Algebra
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about How To Find Ph At The Equivalence Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
