How To Find The Excess Reactant
Muz Play
Mar 21, 2025 · 6 min read
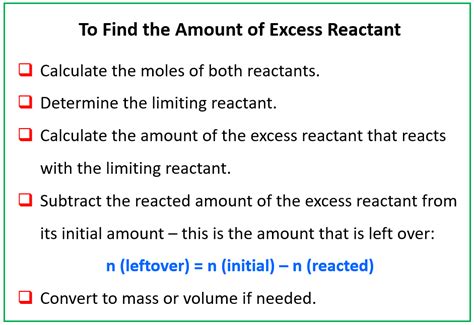
Table of Contents
How to Find the Excess Reactant: A Comprehensive Guide
Determining the excess reactant in a chemical reaction is a crucial step in stoichiometry, allowing us to predict the amount of product formed and understand the efficiency of a reaction. This guide will walk you through the process, explaining the concepts, providing step-by-step examples, and offering tips to avoid common mistakes.
Understanding Reactants and Limiting Reactants
Before diving into identifying the excess reactant, let's clarify some fundamental concepts:
- Reactants: These are the starting materials in a chemical reaction. They are the substances that react with each other to form products.
- Products: These are the substances formed as a result of a chemical reaction.
- Limiting Reactant: This reactant is completely consumed during the reaction. It determines the maximum amount of product that can be formed. Once the limiting reactant is used up, the reaction stops.
- Excess Reactant: This reactant is present in a larger amount than required to react completely with the limiting reactant. Some of it will remain unreacted after the reaction is complete.
Understanding the limiting reactant is essential because it dictates the theoretical yield of the reaction. The theoretical yield is the maximum amount of product that can be produced based on the stoichiometry of the balanced chemical equation and the amount of limiting reactant.
Steps to Identify the Excess Reactant
To find the excess reactant, follow these steps:
-
Balance the Chemical Equation: Ensure the chemical equation representing the reaction is correctly balanced. This ensures the correct mole ratios between reactants and products. A balanced equation is crucial for accurate stoichiometric calculations.
-
Convert Grams to Moles: Convert the given masses of each reactant into moles using their respective molar masses. Remember, the molar mass is the mass of one mole of a substance and is found on the periodic table for elements or calculated from the formula for compounds.
-
Determine the Mole Ratio: Use the coefficients from the balanced chemical equation to determine the mole ratio between the reactants. This ratio indicates the proportion in which the reactants react.
-
Identify the Limiting Reactant: Compare the mole ratio of the reactants to the actual mole ratio calculated in step 2. The reactant that produces the least amount of product based on its available moles and the stoichiometric ratio is the limiting reactant.
-
Calculate the Moles of Excess Reactant Used: Once the limiting reactant is identified, calculate how many moles of the excess reactant are needed to react completely with the limiting reactant. Use the mole ratio from the balanced chemical equation.
-
Calculate the Moles of Excess Reactant Remaining: Subtract the moles of excess reactant used (from step 5) from the initial moles of the excess reactant (from step 2). The result is the number of moles of excess reactant that remain unreacted.
-
Convert Moles to Grams (Optional): If required, convert the remaining moles of excess reactant back into grams using its molar mass. This gives the mass of the excess reactant left over.
Example Problem: Finding the Excess Reactant
Let's illustrate this process with an example. Consider the reaction between hydrogen gas (H₂) and oxygen gas (O₂) to produce water (H₂O):
2H₂(g) + O₂(g) → 2H₂O(l)
Suppose we have 10.0 grams of hydrogen gas and 100.0 grams of oxygen gas. Let's find the excess reactant.
Step 1: Balanced Equation
The equation is already balanced.
Step 2: Convert Grams to Moles
- Moles of H₂: 10.0 g H₂ × (1 mol H₂ / 2.02 g H₂) = 4.95 mol H₂
- Moles of O₂: 100.0 g O₂ × (1 mol O₂ / 32.00 g O₂) = 3.125 mol O₂
Step 3: Determine the Mole Ratio
From the balanced equation, the mole ratio of H₂ to O₂ is 2:1. This means 2 moles of H₂ react with 1 mole of O₂.
Step 4: Identify the Limiting Reactant
- Using H₂: 4.95 mol H₂ × (1 mol O₂ / 2 mol H₂) = 2.475 mol O₂ needed
- Since we only have 3.125 mol O₂, H₂ is the limiting reactant.
Step 5: Calculate Moles of Excess Reactant Used
The moles of O₂ used are 2.475 mol (calculated in step 4).
Step 6: Calculate Moles of Excess Reactant Remaining
Moles of O₂ remaining = Initial moles of O₂ - Moles of O₂ used = 3.125 mol - 2.475 mol = 0.65 mol O₂
Step 7: Convert Moles to Grams (Optional)
Mass of O₂ remaining = 0.65 mol O₂ × (32.00 g O₂ / 1 mol O₂) = 20.8 g O₂
Therefore, oxygen (O₂) is the excess reactant, and 20.8 grams of oxygen will remain unreacted after the reaction is complete.
Advanced Considerations and Common Mistakes
While the steps above provide a clear method, let's address some more complex scenarios and common pitfalls:
-
Reactions with More Than Two Reactants: The process remains the same. You'll need to compare the amount of product formed from each reactant to identify the limiting reactant and subsequently determine the excess reactants.
-
Incomplete Reactions: The calculations assume a 100% yield. In reality, many reactions have yields less than 100%. This means less product is formed than theoretically predicted. However, the identification of the limiting and excess reactants remains the same; the amount of product formed is simply less than the theoretical yield.
-
Impure Reactants: If reactants are impure, the actual amount of the reactive substance is less than the total mass. You need to account for the purity before starting the calculations. For example, if a reactant is only 90% pure, you only have 90% of the mass available for reaction.
-
Incorrect Stoichiometry: Using an unbalanced chemical equation is the most common mistake. Always double-check your equation is balanced before proceeding with the calculations.
-
Unit Conversion Errors: Carefully track units throughout the calculations. Inconsistencies in units will lead to incorrect answers.
Practical Applications of Finding Excess Reactant
Understanding excess reactants has several crucial practical applications in various fields:
-
Chemical Synthesis: In industrial chemical synthesis, using an excess of one reactant can improve the yield of the desired product by pushing the reaction towards completion. This is particularly useful for reactions that are not very efficient or that reach equilibrium before all the limiting reactant is consumed.
-
Chemical Analysis: In analytical chemistry, knowing the excess reactant allows for precise calculations of the amount of analyte present in a sample. Titration experiments are a prime example, where a known excess of a reagent is added, and the remaining excess is determined to calculate the amount of the analyte.
-
Environmental Chemistry: Understanding stoichiometry and excess reactants is crucial in environmental remediation, where the effectiveness of cleanup strategies depends on precise calculations of reactant amounts to remove pollutants effectively.
-
Pharmaceutical Industry: In pharmaceutical manufacturing, precise control over reactant amounts is critical to ensure product purity and consistency. Excess reactants can be carefully chosen to minimize side reactions and improve product quality.
-
Materials Science: Excess reactants influence the properties of materials during synthesis. Precise control of stoichiometry can tailor the material's final properties, like its crystal structure, conductivity, or mechanical strength.
Conclusion
Identifying the excess reactant is a fundamental skill in stoichiometry with wide-ranging applications in chemistry and related fields. By following the step-by-step procedure outlined here, paying close attention to detail, and avoiding common pitfalls, you'll be able to confidently determine the excess reactant in any chemical reaction and utilize this knowledge for a deeper understanding of chemical processes. Remember to always practice and work through various examples to solidify your understanding. The more practice you have, the more comfortable and efficient you will become at performing these critical stoichiometric calculations.
Latest Posts
Latest Posts
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
-
Divides The Body Into Anterior And Posterior Portions
Mar 27, 2025
-
Claim Of Fact Value And Policy
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Excess Reactant . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
