How To Get Molar Mass From Density
Muz Play
Mar 22, 2025 · 6 min read
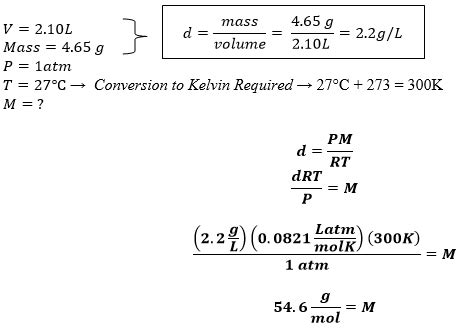
Table of Contents
How to Get Molar Mass from Density: A Comprehensive Guide
Determining molar mass is a fundamental task in chemistry, crucial for various applications, from stoichiometric calculations to understanding material properties. While various methods exist, knowing how to calculate molar mass from density offers a unique and practical approach, particularly useful when dealing with pure substances in their solid or liquid states. This comprehensive guide delves into the theoretical background, step-by-step procedures, and practical considerations involved in this calculation. We'll explore different scenarios and address potential challenges, empowering you with the knowledge to confidently tackle this important aspect of chemistry.
Understanding the Relationship Between Density and Molar Mass
Before diving into the calculations, let's establish the fundamental relationship between density and molar mass. Density (ρ), often expressed in g/cm³, is defined as the mass (m) of a substance per unit volume (V):
ρ = m/V
Molar mass (M), usually expressed in g/mol, represents the mass of one mole of a substance. One mole contains Avogadro's number (6.022 x 10²³) of particles (atoms, molecules, or ions). The connection between density and molar mass arises when we consider the volume occupied by one mole of a substance. This volume is often referred to as the molar volume (Vm).
Vm = V/n
Where 'n' is the number of moles. Combining these equations, we can derive a powerful relationship:
M = ρVm
This equation forms the foundation for calculating molar mass from density. However, determining the molar volume (Vm) requires further consideration depending on the state of the substance (solid, liquid, or gas).
Calculating Molar Mass from Density: Solid and Liquid States
For solids and liquids, the molar volume (Vm) is less straightforward to directly measure than for gases. Instead, we often utilize the crystal structure (for solids) or other experimental data to determine the molar volume indirectly.
Method 1: Using Crystallographic Data (Solids)
Many crystalline solids have well-defined crystal structures determined through techniques like X-ray diffraction. Knowing the unit cell dimensions and the number of formula units per unit cell allows us to calculate the volume occupied by one mole of the substance.
1. Determine the Unit Cell Volume: The unit cell volume (Vc) can be calculated based on the unit cell dimensions (a, b, c for orthogonal systems) using the appropriate formula. For example, for a cubic unit cell, Vc = a³.
2. Determine the Number of Formula Units per Unit Cell (Z): This value is determined from the crystal structure analysis.
3. Calculate the Molar Volume (Vm): Vm = Vc * Nₐ / Z, where Nₐ is Avogadro's number.
4. Calculate the Molar Mass (M): M = ρVm
Example: Consider a cubic crystal with a = 4 Å (4 x 10⁻⁸ cm), Z = 4, and ρ = 2.7 g/cm³.
- Vc = (4 x 10⁻⁸ cm)³ = 6.4 x 10⁻²³ cm³
- Vm = (6.4 x 10⁻²³ cm³) * (6.022 x 10²³) / 4 = 9.635 cm³/mol
- M = 2.7 g/cm³ * 9.635 cm³/mol = 26 g/mol
Method 2: Using Density and Other Experimental Data (Solids and Liquids)
In cases where crystallographic data isn't available, we might use other experimental techniques to indirectly determine the molar volume. For example:
-
Measurement of Volume: A known mass of the substance is weighed and its volume measured accurately using techniques like water displacement. This allows direct calculation of the density.
-
Determination of Formula: The chemical formula of the substance must be known to relate the mass to the number of moles.
-
Molar Mass Calculation: Once the density and number of moles are known, we can calculate the molar mass using the derived relationship: M = ρV/n
Example: Suppose 10g of a substance occupies 5cm³. The chemical formula suggests a molar mass of approximately 200 g/mol.
- Density (ρ) = 10 g / 5 cm³ = 2 g/cm³
- Number of moles (n) = 10 g / 200 g/mol = 0.05 mol
- Molar Volume (Vm) = 5 cm³/ 0.05 mol = 100 cm³/mol
- Calculated Molar Mass (M) = 2 g/cm³ * 100 cm³/mol = 200 g/mol
Calculating Molar Mass from Density: Gaseous State
For gases, the calculation is significantly simpler due to the ideal gas law. The molar volume of an ideal gas at standard temperature and pressure (STP: 0°C and 1 atm) is approximately 22.4 L/mol.
Method 3: Using the Ideal Gas Law (Gases)
The ideal gas law states:
PV = nRT
Where:
- P = pressure
- V = volume
- n = number of moles
- R = ideal gas constant (0.0821 L atm/mol K)
- T = temperature in Kelvin
At STP, we can simplify this to:
Vm = V/n ≈ 22.4 L/mol
We can then use our fundamental equation:
M = ρVm
To calculate the molar mass, remembering to convert density to g/L to maintain consistent units.
Example: A gas has a density of 1.96 g/L at STP.
- M = 1.96 g/L * 22.4 L/mol = 43.9 g/mol
Important Note: The ideal gas law provides an approximation. Real gases deviate from ideal behavior, especially at high pressures and low temperatures. For more accurate results with real gases, the van der Waals equation or other more sophisticated equations of state should be used.
Practical Considerations and Error Analysis
Accurate determination of molar mass from density requires careful attention to detail and consideration of potential sources of error:
-
Precise Measurement: Accurate measurements of mass and volume are crucial. Using calibrated instruments and employing appropriate techniques are essential for minimizing experimental error.
-
Temperature and Pressure Control: For gases, maintaining constant temperature and pressure is vital, as these factors significantly influence density. Deviations from STP conditions require adjustments to the calculations.
-
Purity of Substance: Impurities in the sample will affect the measured density and subsequently the calculated molar mass. Using pure samples is paramount.
-
State of Matter: The chosen method depends heavily on the state of the matter. Applying methods for solids to liquids or gases will lead to inaccurate results.
-
Ideal Gas Assumption: Remember that the ideal gas law is an approximation. The greater the deviation from ideal behavior, the larger the error in the molar mass calculation.
Conclusion
Determining molar mass from density provides a valuable method for characterizing substances. While the approach varies depending on whether the substance is a solid, liquid, or gas, the underlying principle remains consistent: relating mass, volume, and the number of moles using appropriate equations. By understanding the theoretical background, mastering the calculation procedures, and carefully considering potential sources of error, you can confidently use density measurements to determine molar mass accurately. This skill is essential for various applications in chemistry and material science, from identifying unknown compounds to understanding the properties of materials. Remember to always choose the most appropriate method based on the state of matter and available data, ensuring the highest accuracy in your results.
Latest Posts
Latest Posts
-
Group 4 Elements Of The Periodic Table
Mar 22, 2025
-
The Monomer Of A Protein Is
Mar 22, 2025
-
Groups 3 12 Contain Metals Known As
Mar 22, 2025
-
Which Phase Change Is An Endothermic Change
Mar 22, 2025
-
Determine Whether The Graph Is The Graph Of A Function
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How To Get Molar Mass From Density . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
